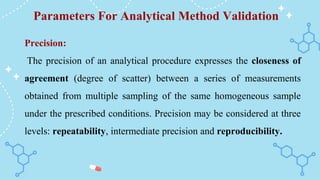
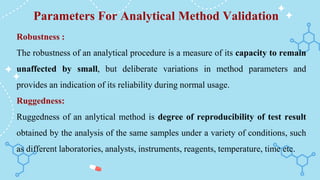
The document outlines the analytical method validation process as defined by regulatory bodies like the FDA and WHO, emphasizing the need for documented evidence to ensure consistency and reliability in analytical testing. It details the steps involved in method validation, key parameters such as accuracy, precision, selectivity, and robustness, as well as scenarios that necessitate validation. Additionally, it includes references and examples of previous examination questions related to the topic.














![1. Explain “Accuracy ” of assay method with example.?
[7.5 marks] april 2022
2. Write a short Note on the LOD and LOQ of the analytical
validation.? (5 Marks) april 2019.
3. Discuss the different parameters of Method Validation as per
ICH Guidelines.
4. What are the steps involved in analytical Method Validation.?
Previous Year Question](https://image.slidesharecdn.com/pvanalyticalvalidation-1-240311133028-258d2fd7/85/Analytical-Methods-Validation-PV-M-Pharmacy-QA-17-320.jpg)
