Quality by Design (QbD) is a systematic approach to pharmaceutical development that begins with predefined objectives and emphasizes product and process understanding based on sound science. The main objectives of QbD are to ensure quality products by combining prior knowledge with new data to identify critical quality attributes and critical process parameters, and establish a control strategy within a design space. This approach helps provide a better understanding of processes and fewer batch failures through improved control and management of changes over the product lifecycle.









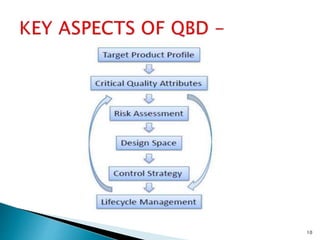
![1] The Target Product Quality Profile (TPQP) -
TPQP has been defined as a “prospective
and dynamic summary of the quality
characteristics of a drug product that ideally
will be achieved to ensure that the desired
quality, and thus the safety and efficacy, of a
drug product is realized”.
11](https://image.slidesharecdn.com/qbdchetanpawar-180730090242/85/INTRODUCTION-TO-QUALITY-BY-DESIGN-QBD-11-320.jpg)

![13
2] Critical Quality Attribute (CQA) -
Once TPQP has been identified, the next step is
to identify the relevant CQAs.
A CQA has been defined as “a physical, chemical,
biological, or microbiological property or
characteristic that should be within an
appropriate limit, range, or distributed to ensure
the desired product quality”
Prior product knowledge, such as the
accumulated laboratory, nonclinical and clinical
experience with a specific product-quality
attribute, is the key in making these risk
assessments](https://image.slidesharecdn.com/qbdchetanpawar-180730090242/85/INTRODUCTION-TO-QUALITY-BY-DESIGN-QBD-13-320.jpg)
![3] Critical Process Parameter (CPPs) -
Critical process parameters (CPPs) are defined
as “parameters whose variability have an
impact on a CQA and therefore should be
monitored or controlled to ensure the
process produces the desired quality”
Process robustness is defined as the ability of
a process to demonstrate acceptable quality
and performance and tolerate variability in
inputs at the same time.
14](https://image.slidesharecdn.com/qbdchetanpawar-180730090242/85/INTRODUCTION-TO-QUALITY-BY-DESIGN-QBD-14-320.jpg)
![4] Risk Assessment -
Quality risk management is a systematic
process for the assessment, control,
communication and review of risks to the
quality of the drug (medicinal) product across
the product lifecycle.
The initial list of potential parameters which
can affect CQAs can be quite extensive but
can be reduced by quality risk assessment
(QRA).
15](https://image.slidesharecdn.com/qbdchetanpawar-180730090242/85/INTRODUCTION-TO-QUALITY-BY-DESIGN-QBD-15-320.jpg)
![16
5] Design Space –
The ICH Q8(R2) States that the design space
is multi dimensional combination and
interaction of input variables (e.g., material
attributes) and process parameters that have
been demonstrated to provide assurance of
quality.
Working within the design space is not
considered as a change. Movement out of the
design space is considered to be a change
and would normally initiate a regulatory post
approval change process .](https://image.slidesharecdn.com/qbdchetanpawar-180730090242/85/INTRODUCTION-TO-QUALITY-BY-DESIGN-QBD-16-320.jpg)
![6] Control Strategy-
Control strategy is defined as “a planned set of
controls, derived from current product and process
understanding that assures process performance and
product quality”.
The ability to evaluate and ensure the quality of in-
process and/or final product based on process data
which typically include a valid combination of
measured material attributes and process controls.
ICH Q8(R2).
The control strategy can include the following
elements: procedural controls, in process controls, lot
release testing, process monitoring, characterization
testing, comparability testing and stability testing .
17](https://image.slidesharecdn.com/qbdchetanpawar-180730090242/85/INTRODUCTION-TO-QUALITY-BY-DESIGN-QBD-17-320.jpg)
![7] Life Cycle Management
In the QBD paradigm, process changes within
the design space will not require review or
approval.
Therefore, process improvements during the
product life cycle with regard to process
consistency and throughput could take place
with fewer post approval submissions.
18](https://image.slidesharecdn.com/qbdchetanpawar-180730090242/85/INTRODUCTION-TO-QUALITY-BY-DESIGN-QBD-18-320.jpg)