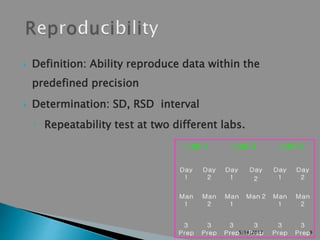
The document outlines the validation parameters for analytical methods as per FDA and ICH guidelines, highlighting specificity, linearity, accuracy, precision, limit of detection, and robustness. It distinguishes between validation concepts in ICH and USP, emphasizing the necessity of methods being suitable for intended use. Key terms and definitions related to these parameters are provided, along with specific criteria for assay accuracy and precision in various tests.