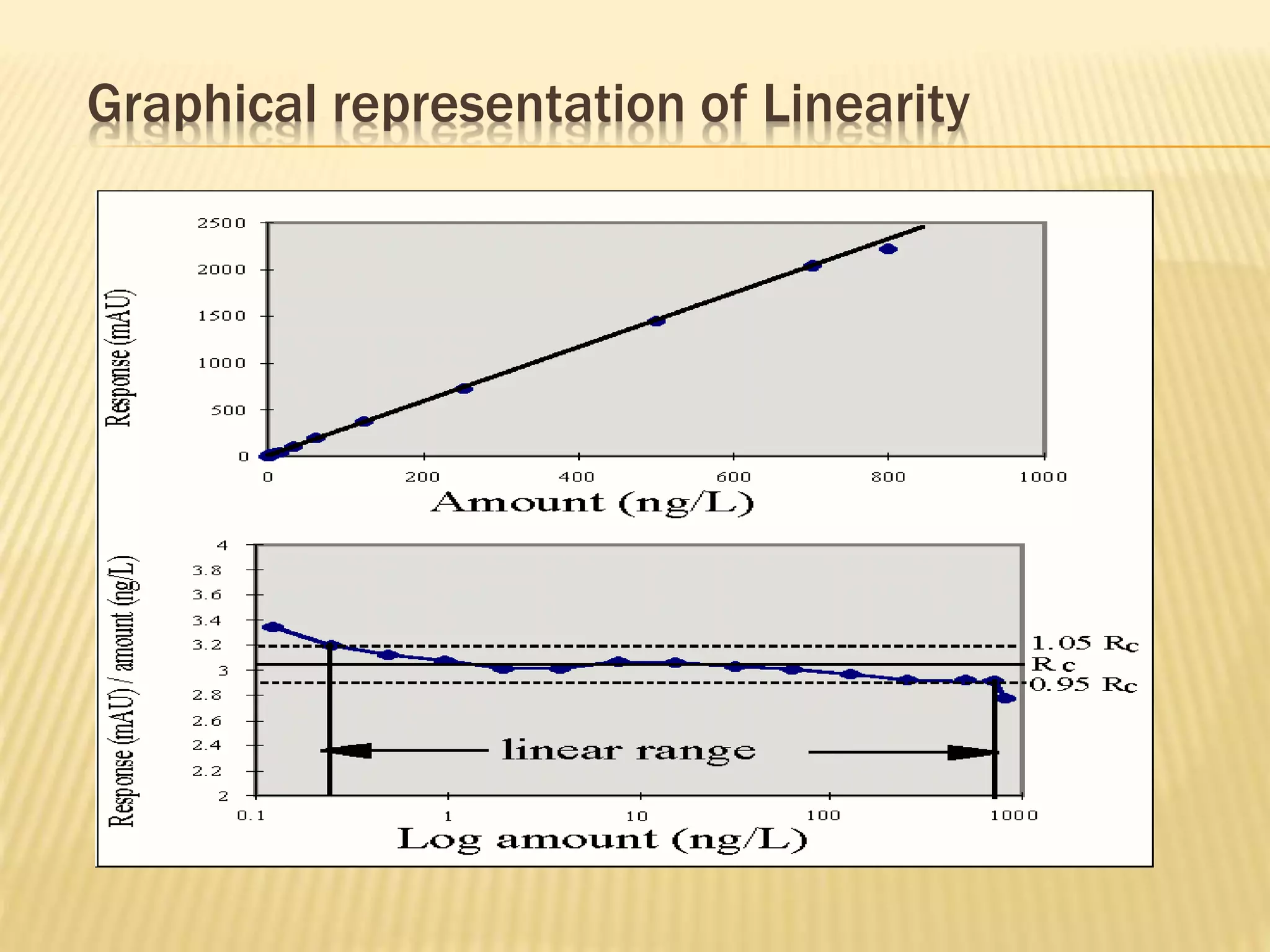
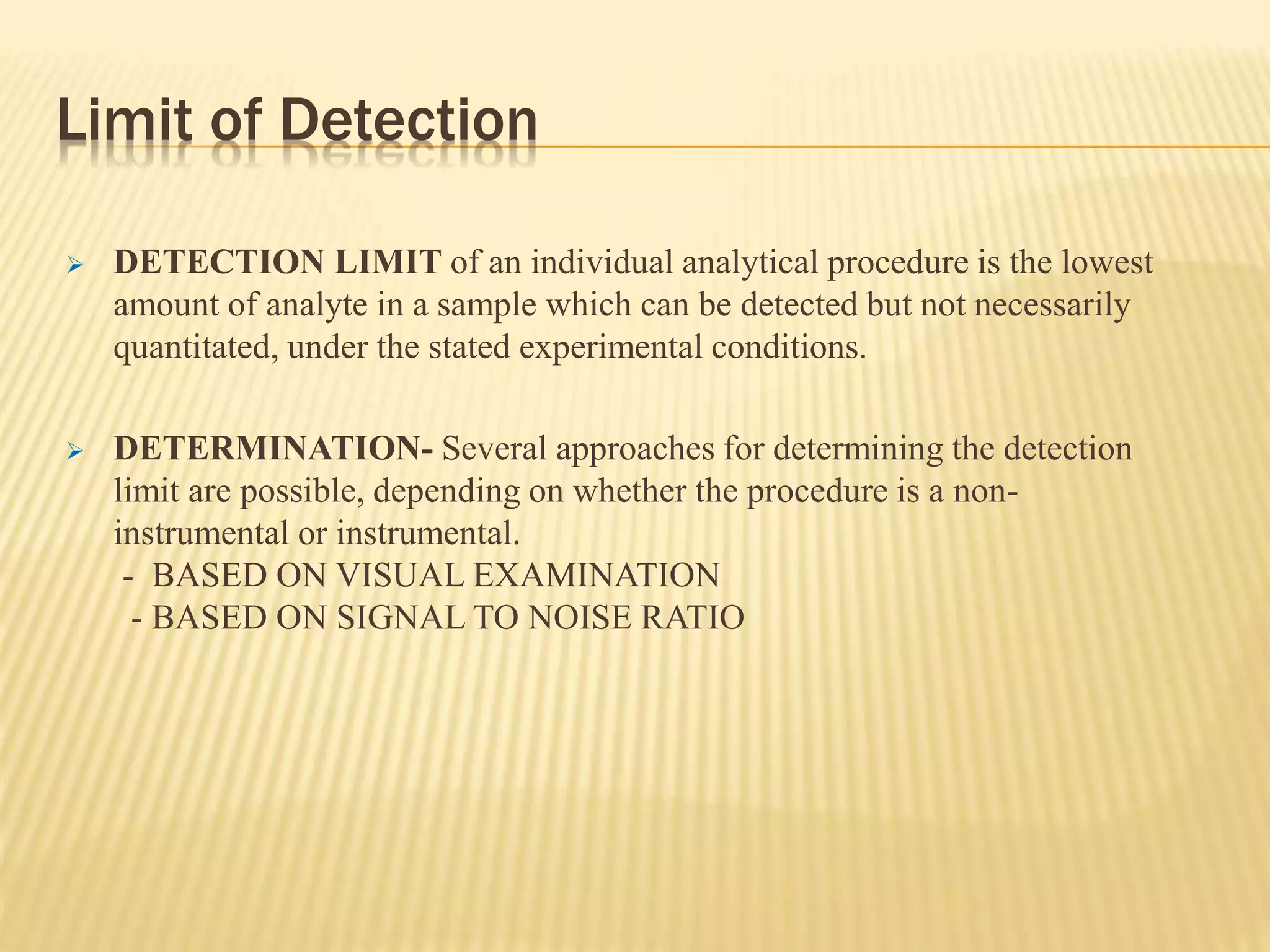
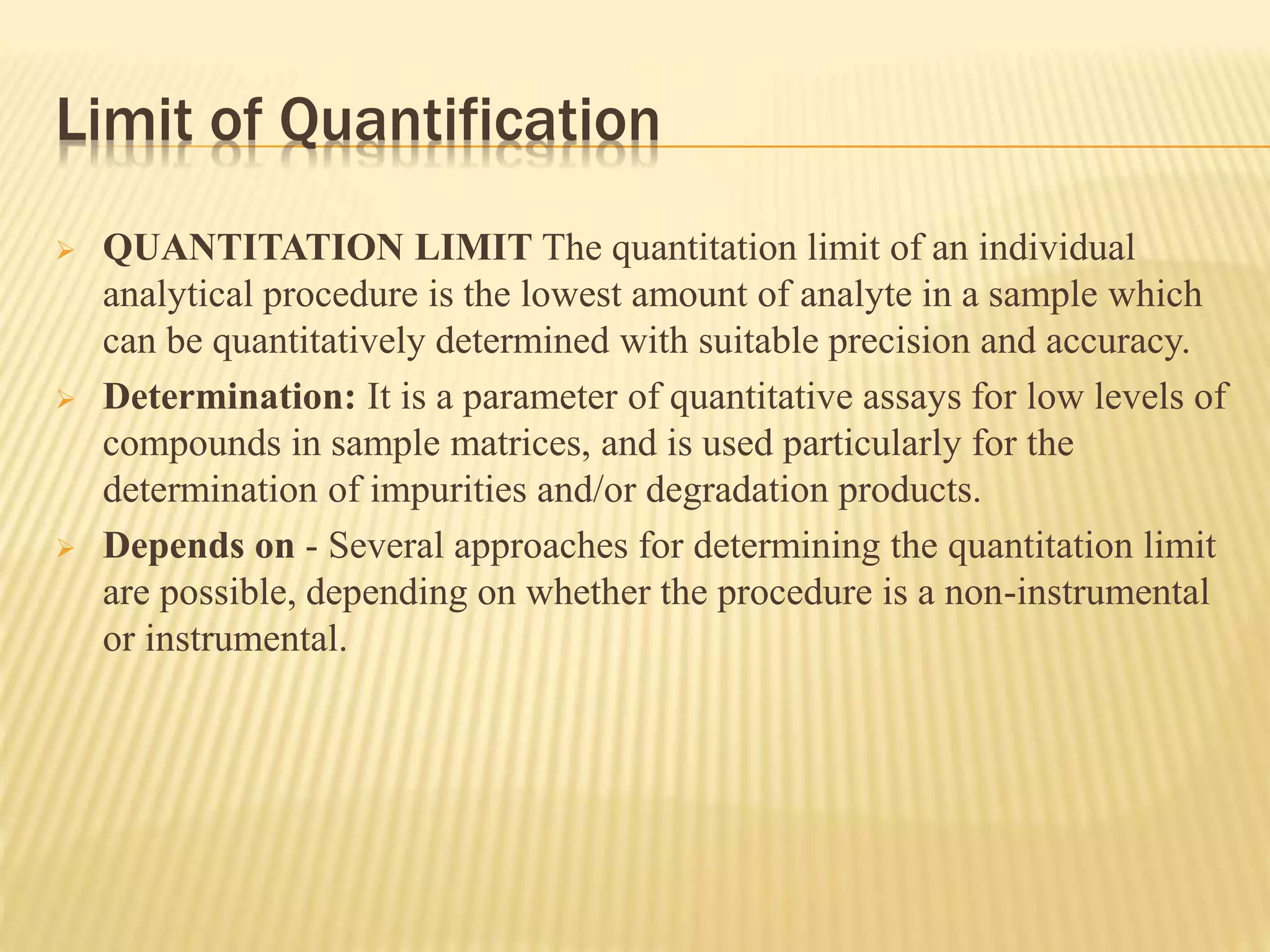
The document discusses process validation in the pharmaceutical industry. It defines process validation as ensuring a manufacturing process is capable of consistently producing a product meeting its predetermined specifications and quality attributes. The summary describes the three stages of process validation: process design, process qualification, and continued process verification. Key parameters that must be validated for analytical methods used in process validation are also listed, including specificity, linearity, accuracy, precision, limits of detection and quantification, and heat sensitivity.