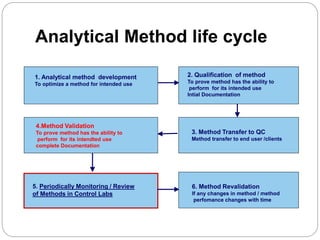
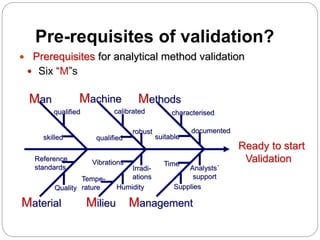
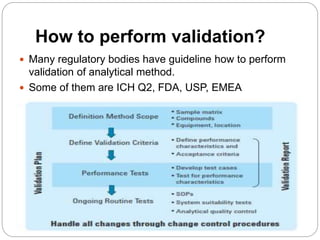
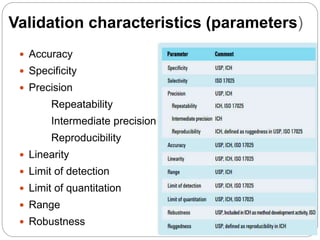
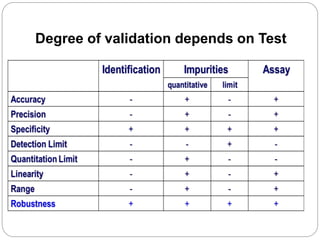
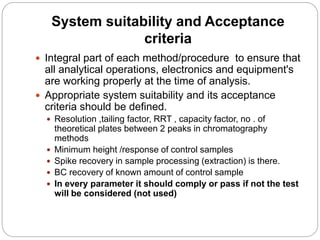
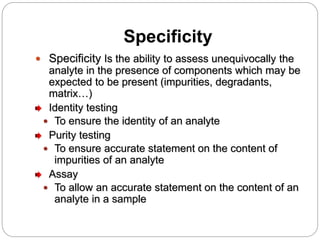
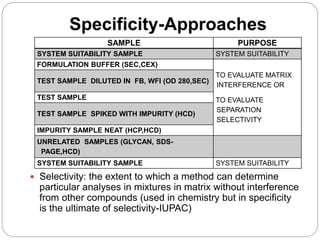
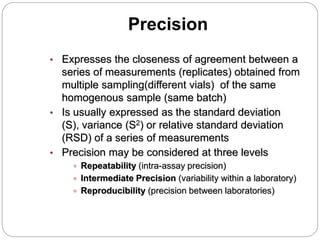
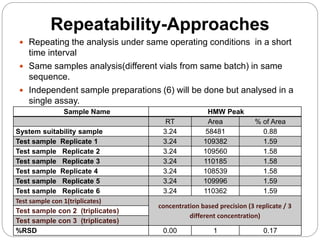
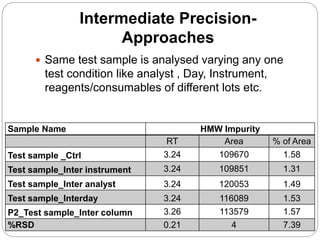
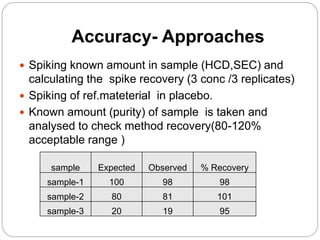
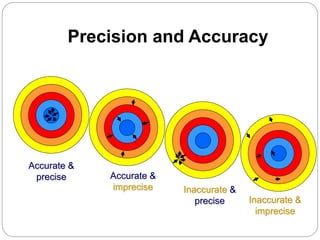
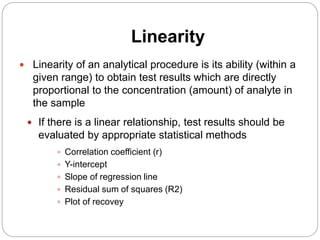
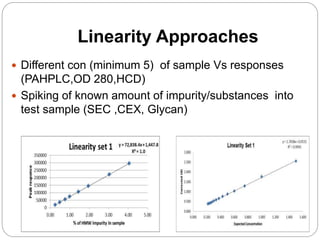
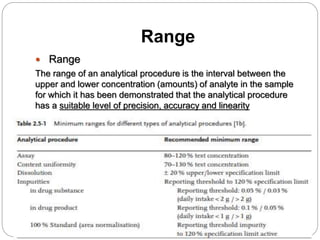
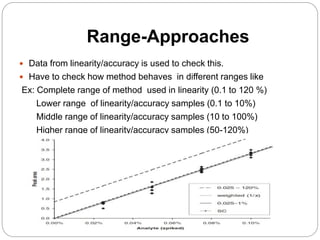
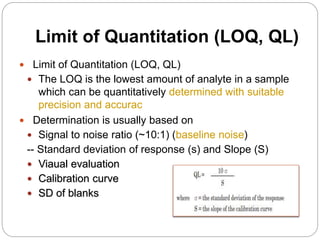
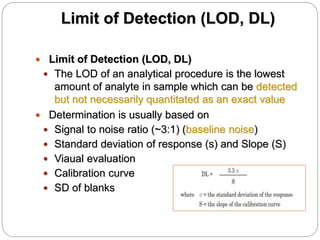
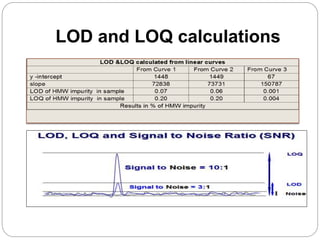
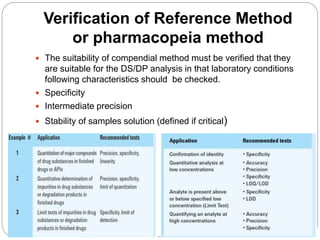
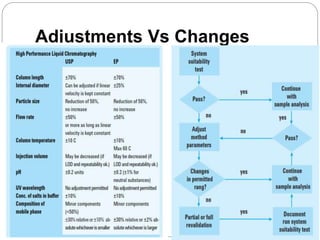
The document discusses the analytical method validation process, highlighting its importance for ensuring the reliability and compliance of analytical methods used in pharmaceuticals. It outlines the stages of validation, including method development, qualification, transfer, monitoring, and revalidation, along with key parameters such as accuracy, precision, specificity, and robustness. Additionally, the document references various regulatory guidelines that inform best practices in method validation.