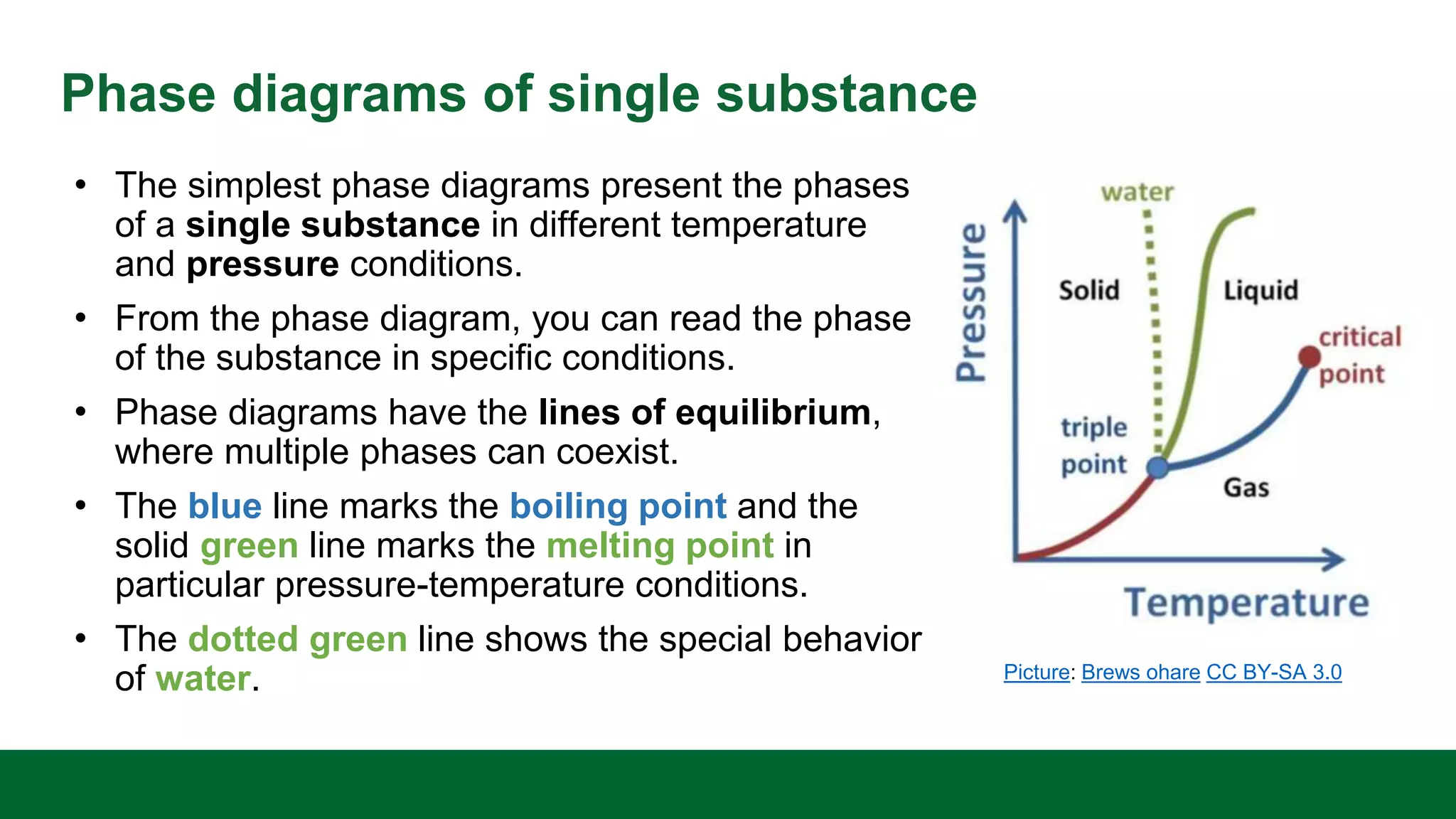
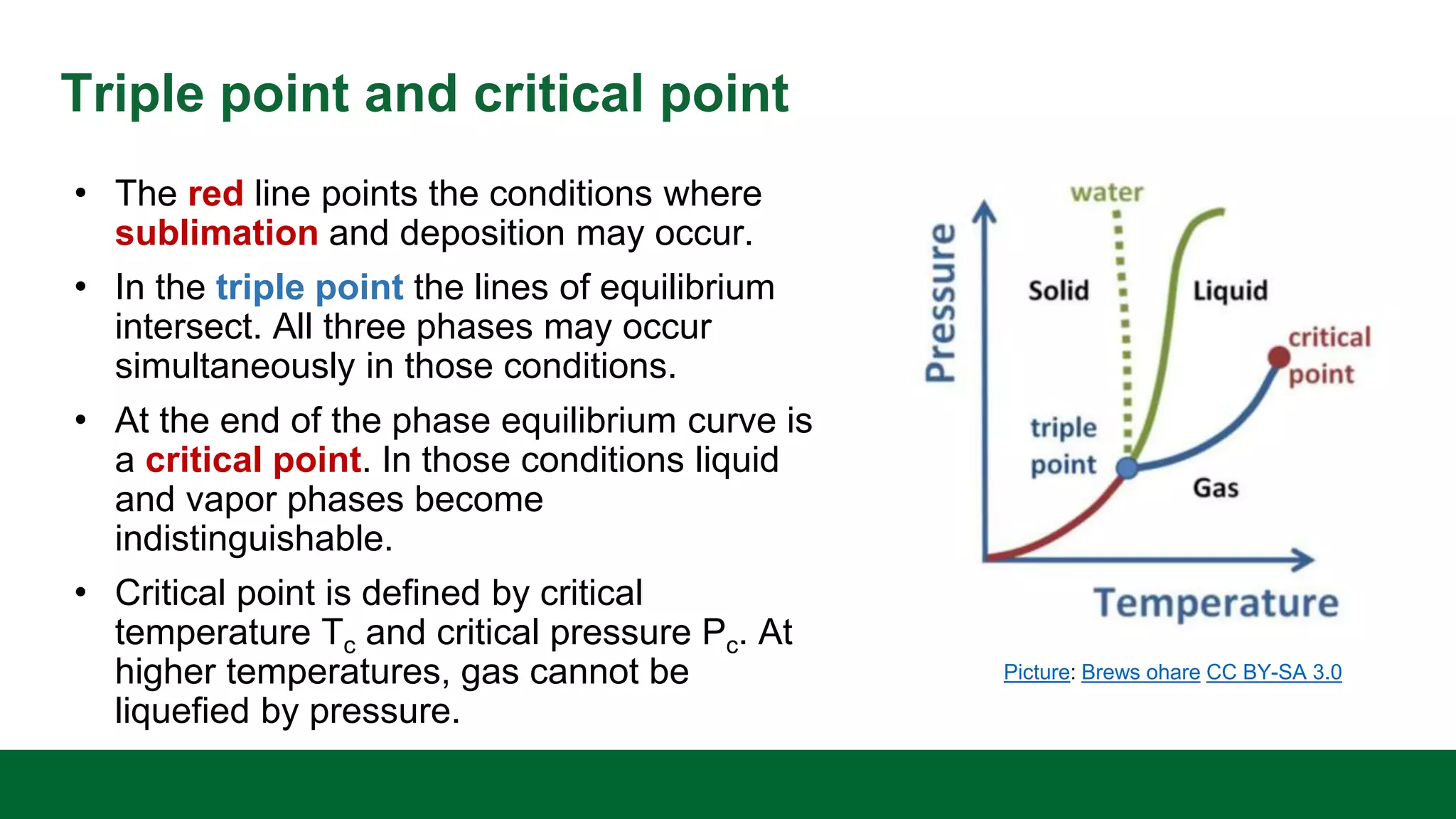
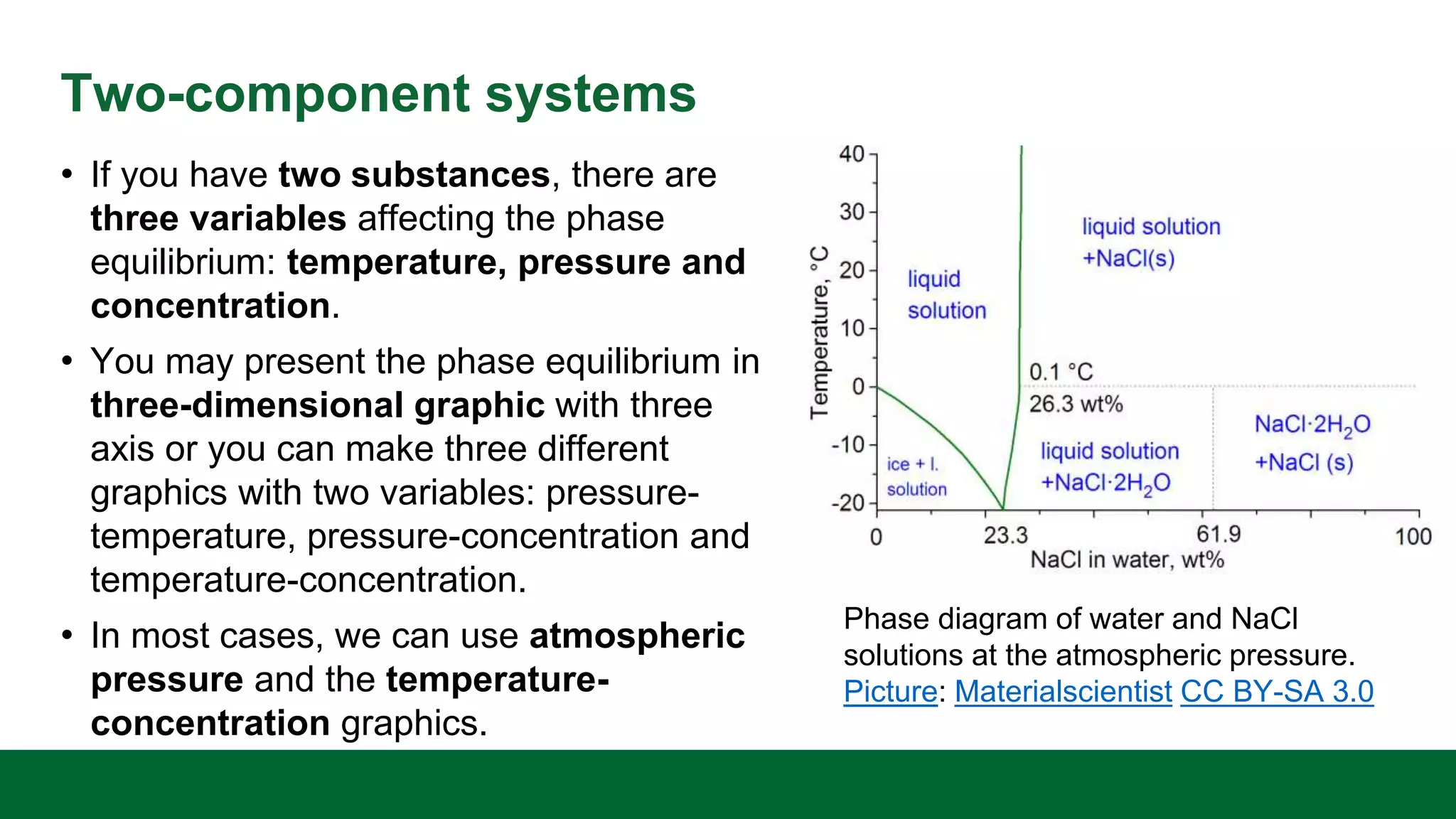
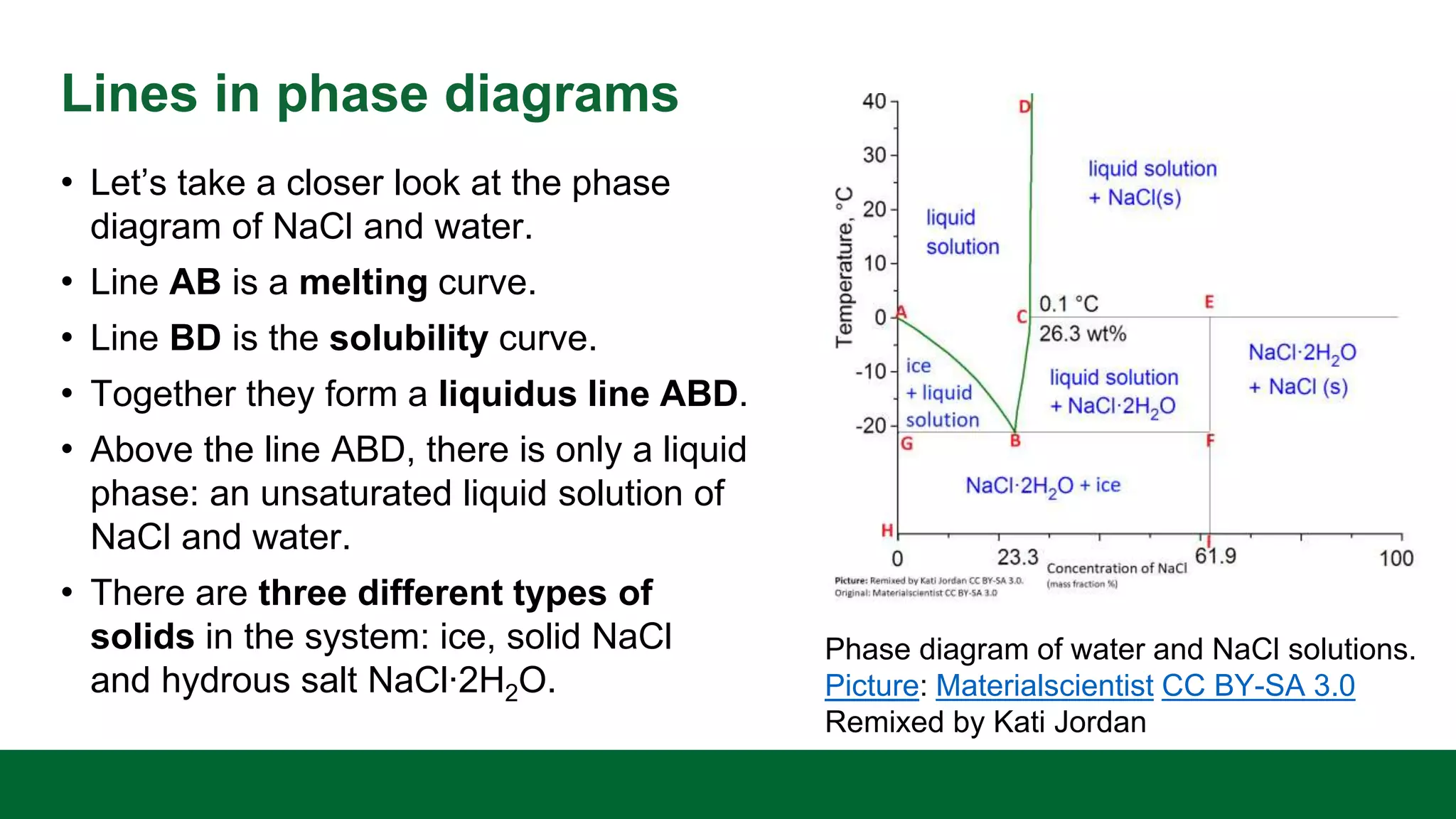
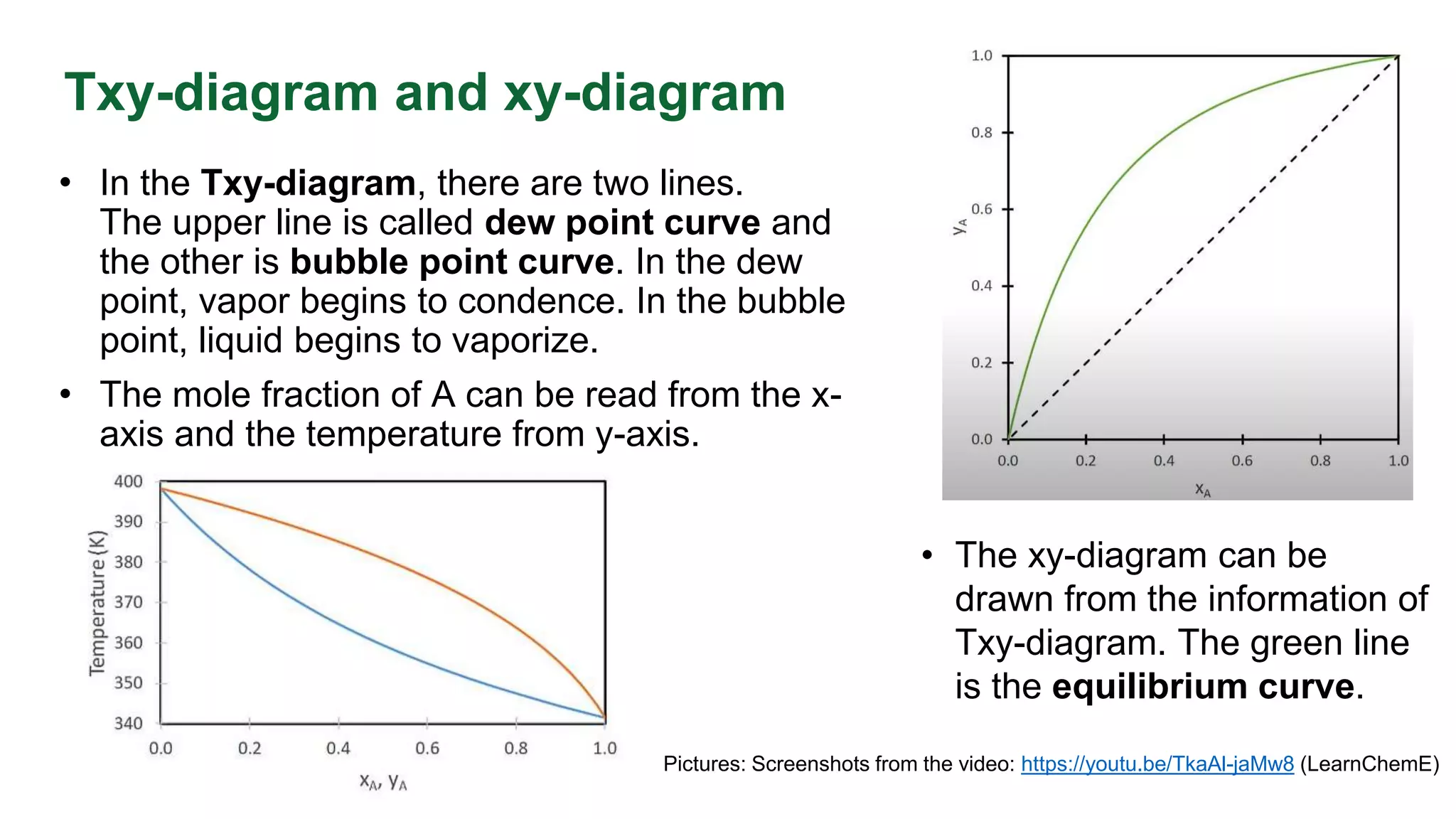
This document discusses the key differences between equilibrium and rate in mass transfer operations. It explains that equilibrium sets the maximum amount that can be transferred, while rate depends on driving force, area, and resistance. Various mass transfer processes are modeled depending on if they reach equilibrium (distillation) or involve diffusion (membranes). Rate equations and ways to increase rate are presented. Phase diagrams for single and multiple component systems are also covered, including lines, points, and how to read information from them. Gibbs phase rule and its application to distillation with two components and phases is explained.