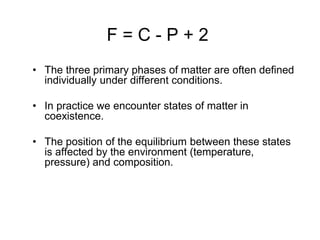
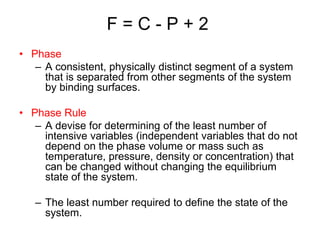
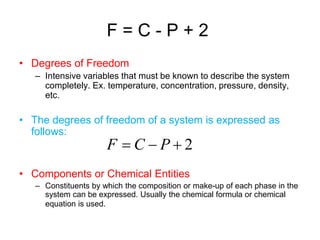
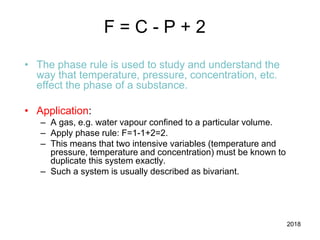
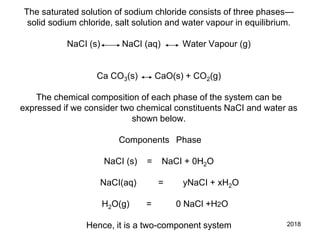
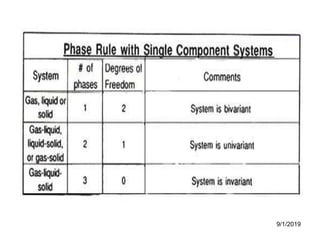
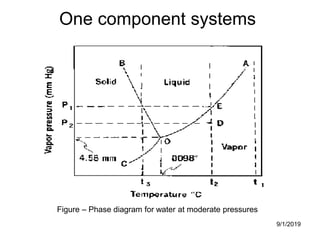
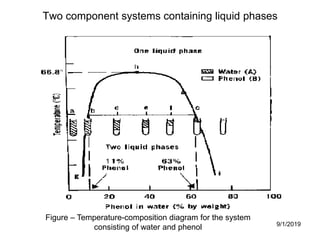
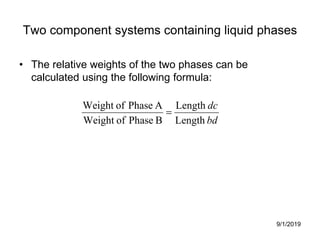
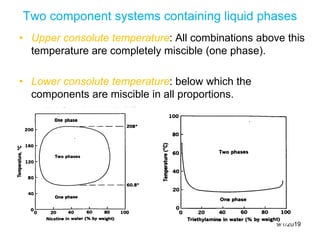
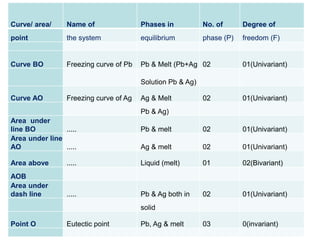
The document discusses the phase rule and its application to various systems. It begins by defining key terms like phase, degrees of freedom, and the phase rule equation. It then provides examples of applying the phase rule to one-component systems like water, two-component liquid systems like phenol-water, and condensed systems like lead-silver alloys. Specific points discussed include vapor-liquid-solid equilibrium, critical solution temperatures, tie lines in partially miscible systems, and identifying invariant, univariant and bivariant regions in phase diagrams.