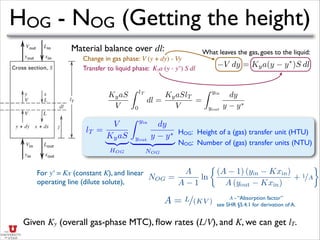
- This document describes absorption and stripping processes using packed columns and graphical methods.
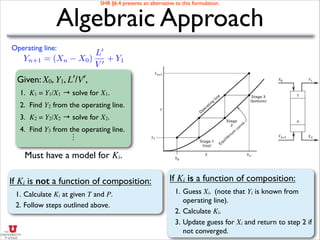
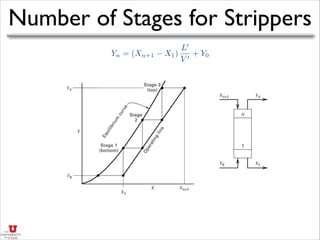
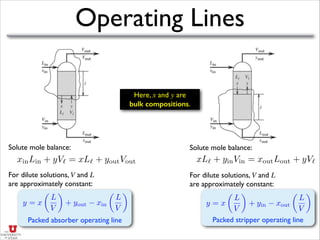
- It discusses operating lines, height of transfer units (HOG), number of transfer units (NOG), and how to calculate the height equivalent of a theoretical plate (HETP) for a packed column given mass transfer coefficients, flow rates, and equilibrium data.
- An example is provided to calculate the HETP for a specific packed column based on the mass transfer coefficients, flow rates, and equilibrium constant given.

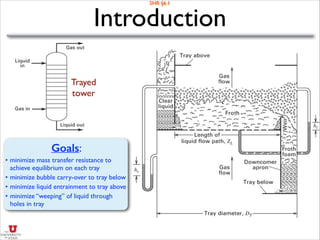








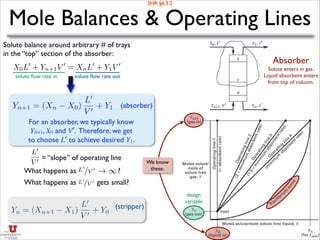
![Absorber: Minimum Flow Rate
Yn+1 = (Xn X0)
L0
V 0
+ Y1
Absorber
Solute enters in gas.
Liquid absorbent enters
from top of column.
L0
=
V 0
(YN+1 Y1)
XN X0
Over the whole tower (n=N):
L′min corresponds to equilibrium
with XN and YN+1.
KN =
yN+1
xN
=
YN+1/(1+YN+1)
XN/(1+XN )
For dilute solutes (Y ≈ y and X ≈ x):
If X0 ≈ 0 then:
L0
min = V 0
✓
yN+1 y1
yN+1/KN x0
◆
Corresponding
equation for a
stripper:
L0
min =
V 0
(YN+1 Y1)
YN+1/[YN+1(KN 1)+KN ] X0
As V′ ↑, L′min↑.
SHR §6.3.3
Why are these in equilibrium?
L0
min = V 0
KN · (fraction absorbed)
V 0
min =
L0
KN
· (fraction stripped)
This is the “best” we can achieve
given the inlet constraints.](https://image.slidesharecdn.com/absorptionstripping-161122015839/85/Absorption-stripping-12-320.jpg)