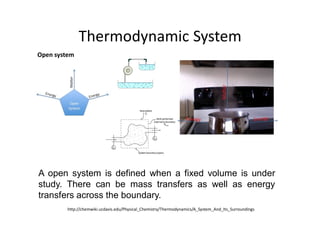
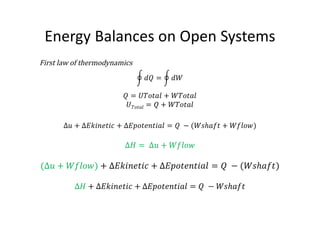
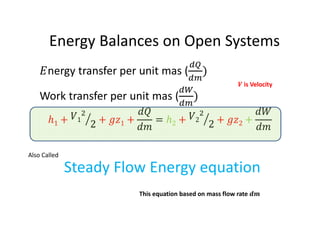
This document discusses energy accounting and energy balances. It defines closed and open systems and derives the steady flow energy equation for open systems. The steady flow energy equation equates the total energy entering and leaving an open system on a mass or time basis. It accounts for changes in kinetic energy, potential energy, heat transfer, and work. The document also discusses the conservation of mass and defines steady and unsteady flow systems.