1) The document discusses phase equilibria and phase diagrams, including definitions of key terms like phase, component, and degrees of freedom.
2) It provides examples of phase diagrams for one-component systems like water, which has three phases (ice, liquid water, vapor) in equilibrium, and sulphur, which has four phases.
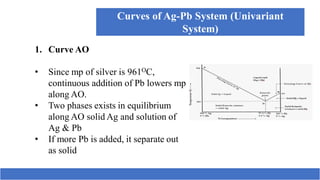
3) Details are given on important features of phase diagrams like melting curves, vaporization curves, triple points, and how to apply Gibbs phase rule to analyze systems.