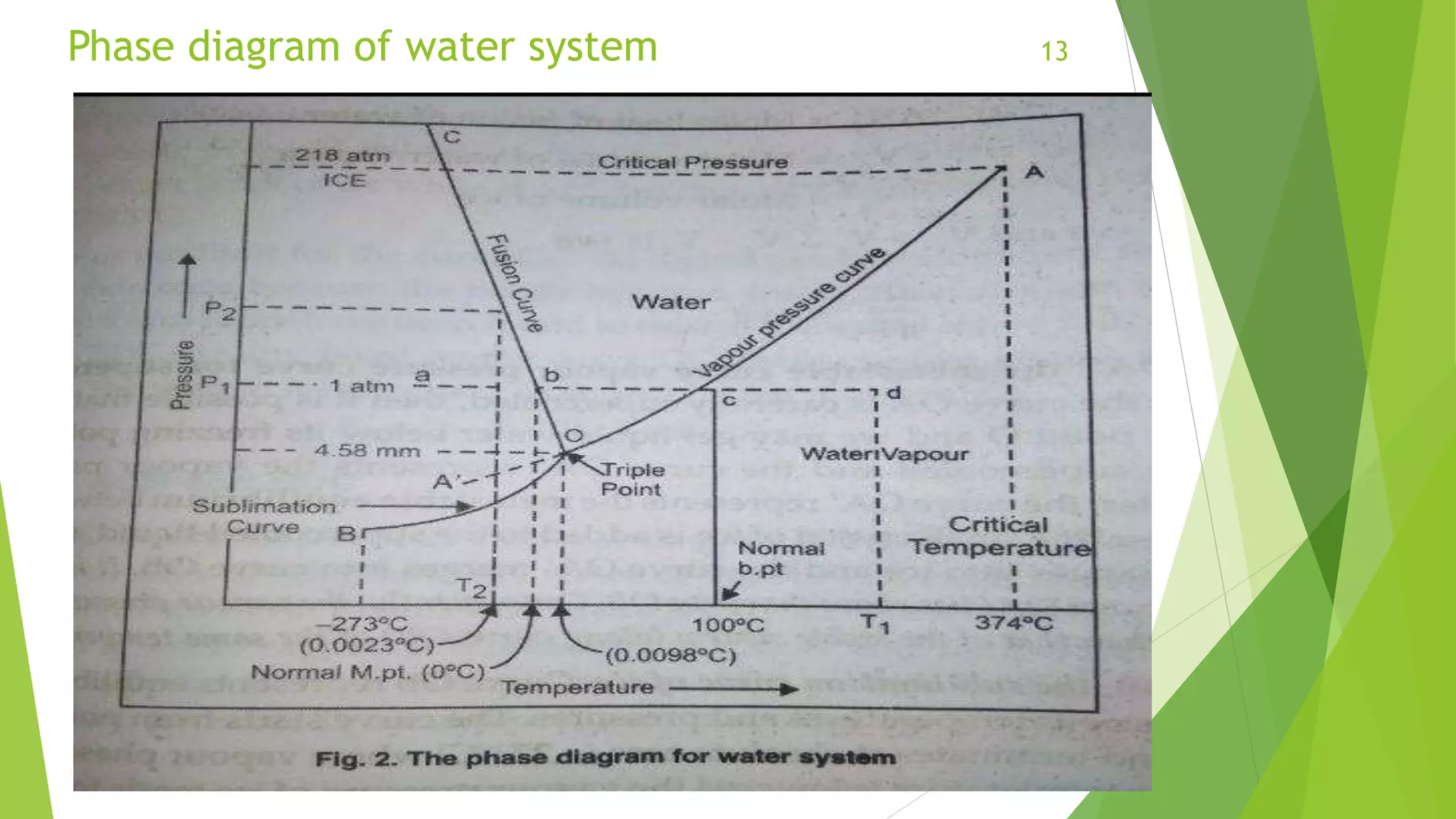
The document provides an overview of phase equilibria, including definitions of phases, components, and degrees of freedom, along with equilibrium types and the conditions necessary for phase equilibrium. It explains Gibbs' phase rule, which mathematically relates the number of phases, components, and degrees of freedom in a system. Additionally, the document discusses the phase diagram of water, illustrating the relationships and conditions under which different phases coexist.







![Cont... 11
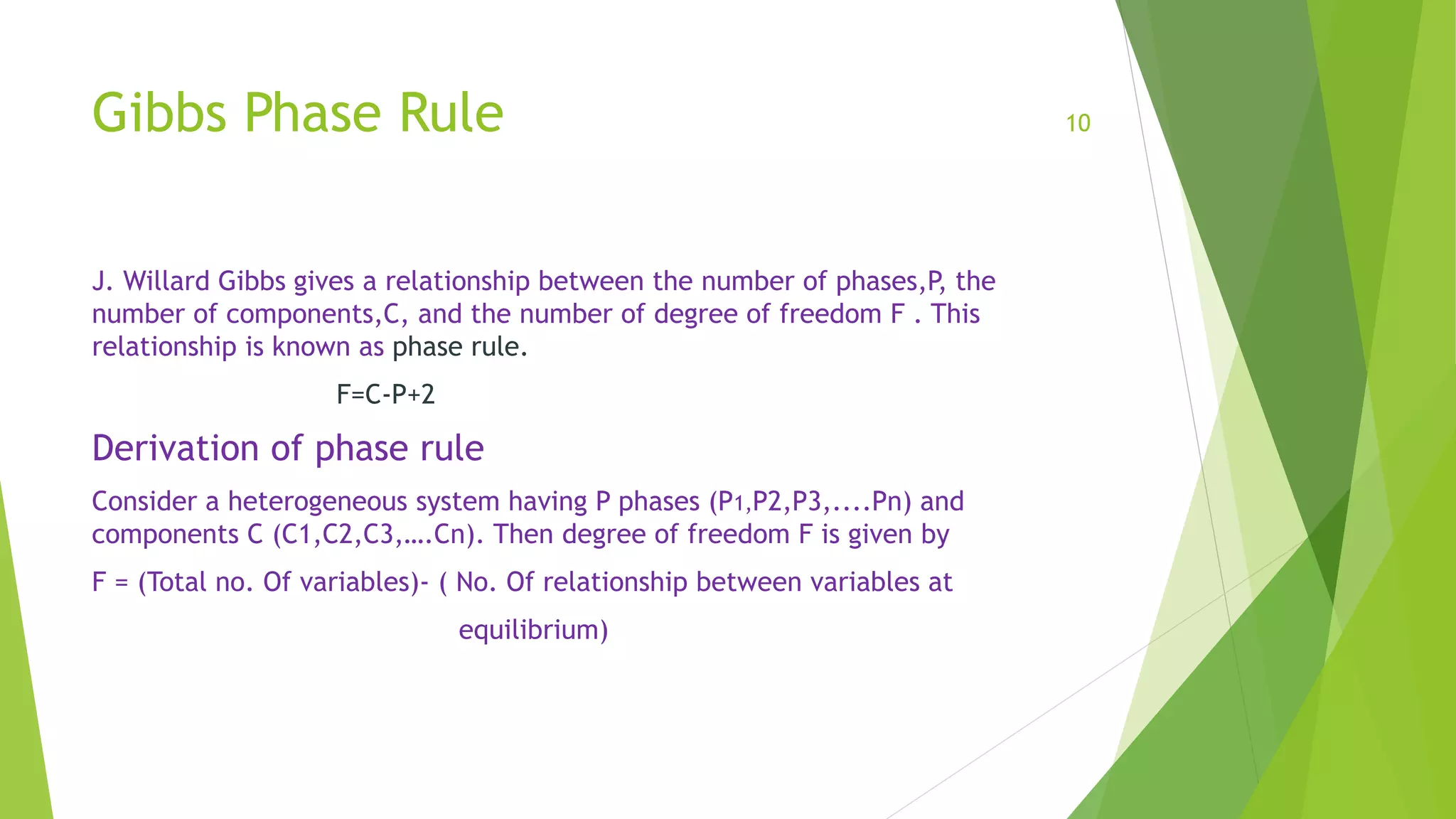
The total number of of variables
1. one is temperature and one is pressure variable for whole system.
2. P(C-1) are composition variables.
Total number of variables = P(C-1) +1+1
= P(C-1)+2
. Number of relationship between variables at equilibrium
C(P-1)
Thus degree of freedom = [P(C-1)+2]-[C(P-1)] = PC-P+2-PC+C = C-P+2
This Is the Gibbs phase rule.](https://image.slidesharecdn.com/meenakshikhatkar-191111134445/75/Phase-equilibria-by-Meenakshi-11-2048.jpg)