Embed presentation
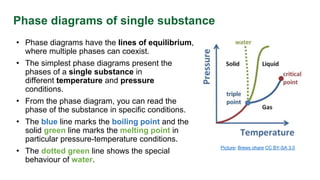
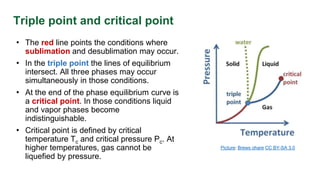
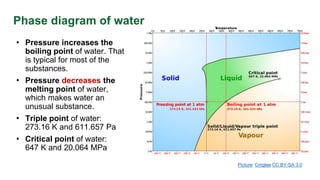
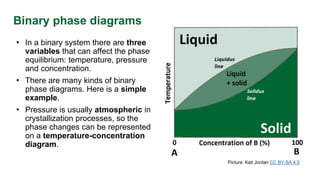
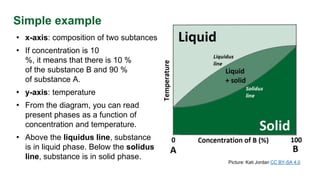

This document discusses phase diagrams and their use in understanding the phases of single and multiple substances under different temperature and pressure conditions. It explains key points such as lines of equilibrium, triple points, critical points, and how to read binary phase diagrams showing the phases of systems with two components as a function of temperature and concentration. Examples of phase diagrams are provided for water and a simple binary system.






