Embed presentation


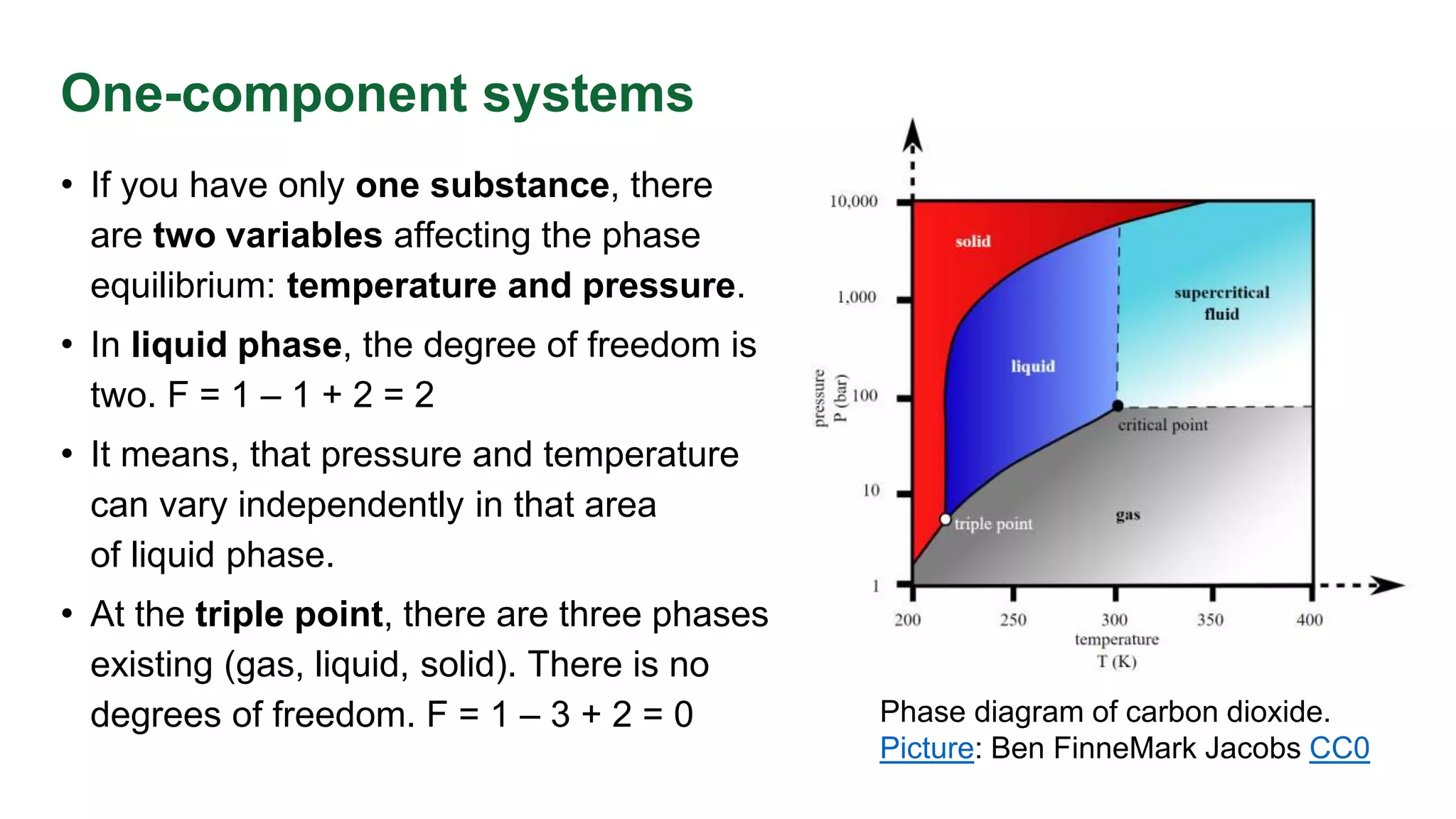
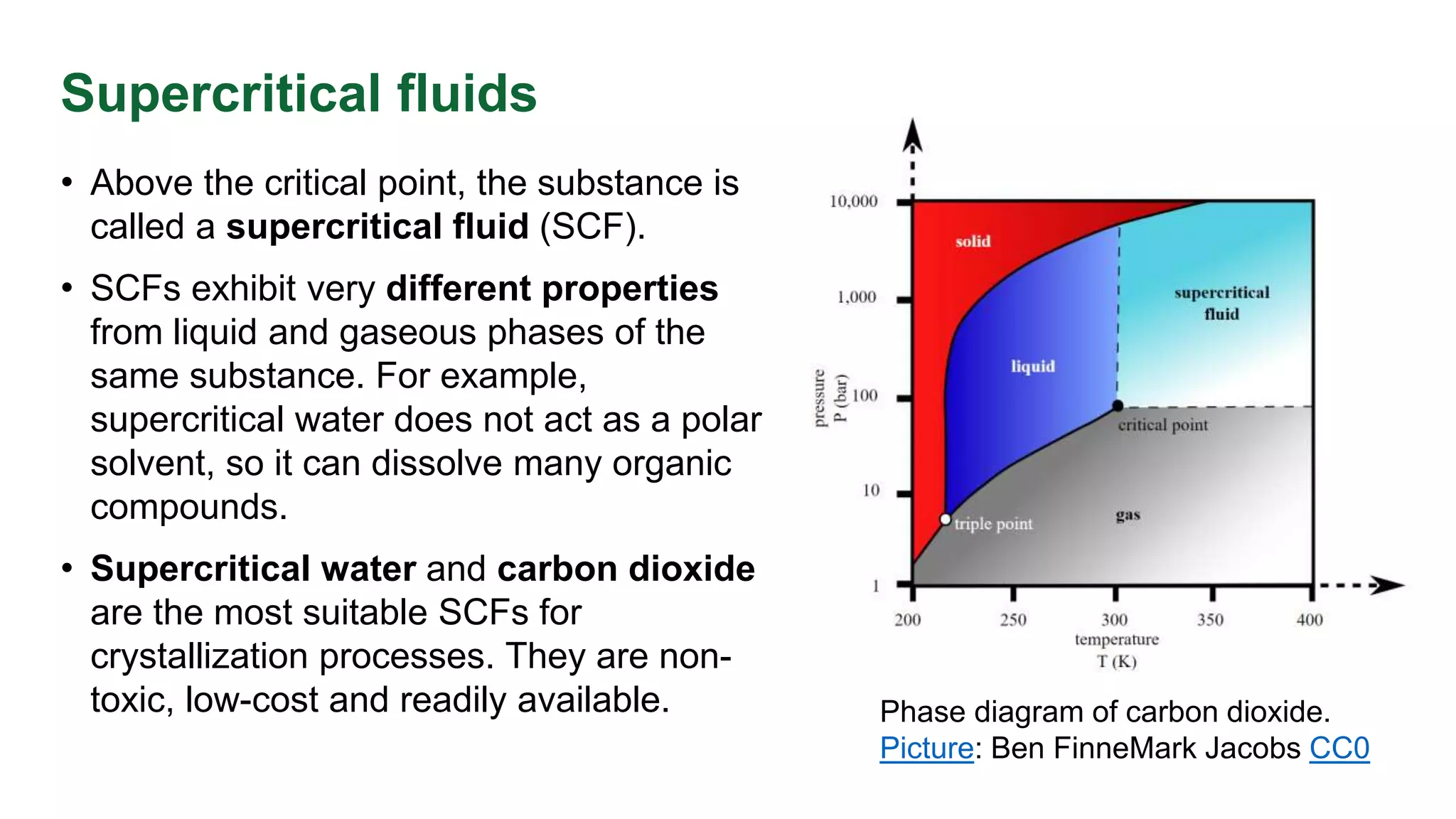
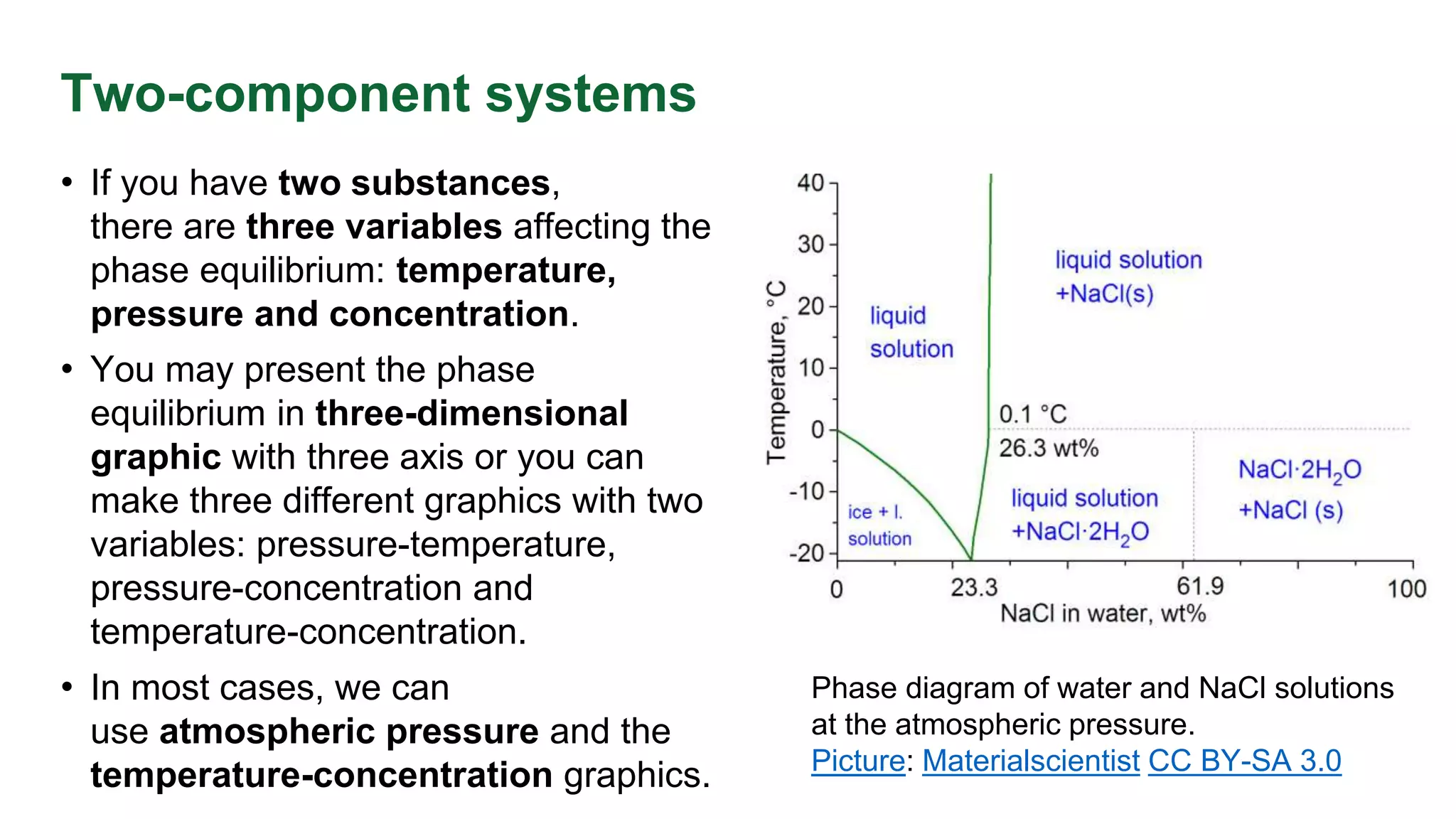

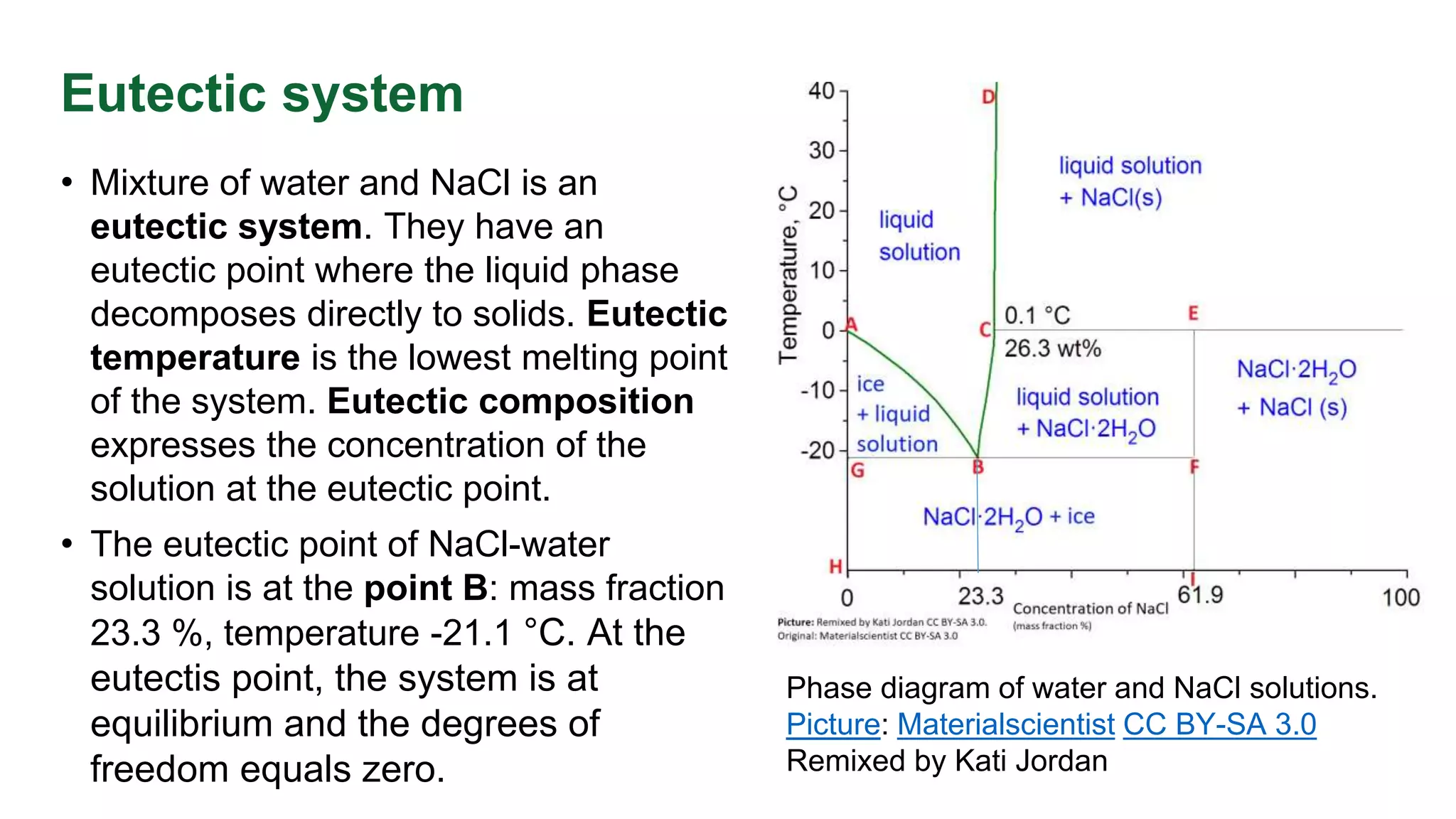


This document discusses phase equilibrium and Gibbs' phase rule, which defines the relationship between degrees of freedom, components, and phases in a system. It highlights the differences between one-component and two-component systems, focusing on supercritical fluids and the eutectic system of NaCl and water. The document also includes graphical representations of phase diagrams and relevant temperatures and compositions at equilibrium points.








