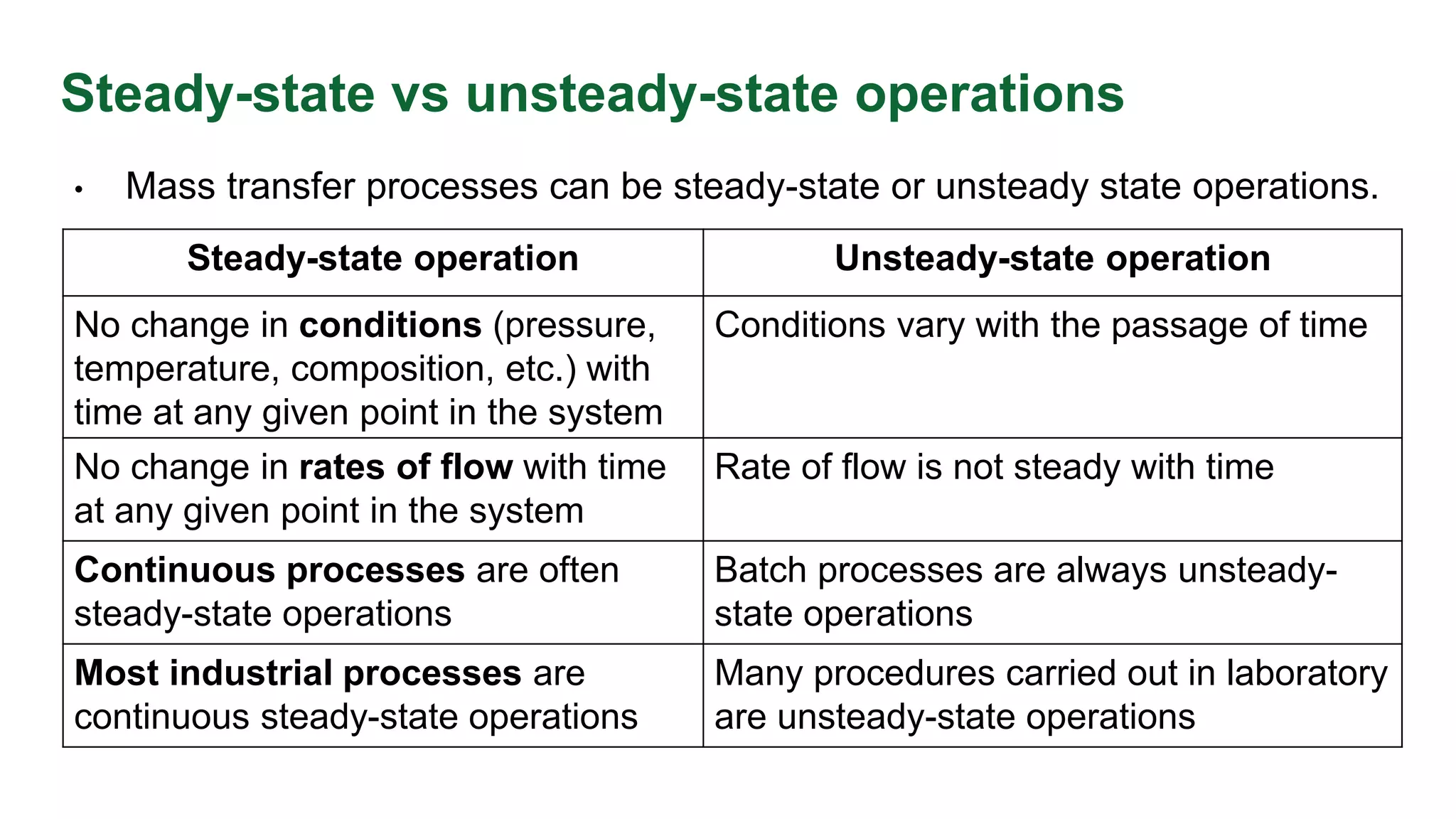
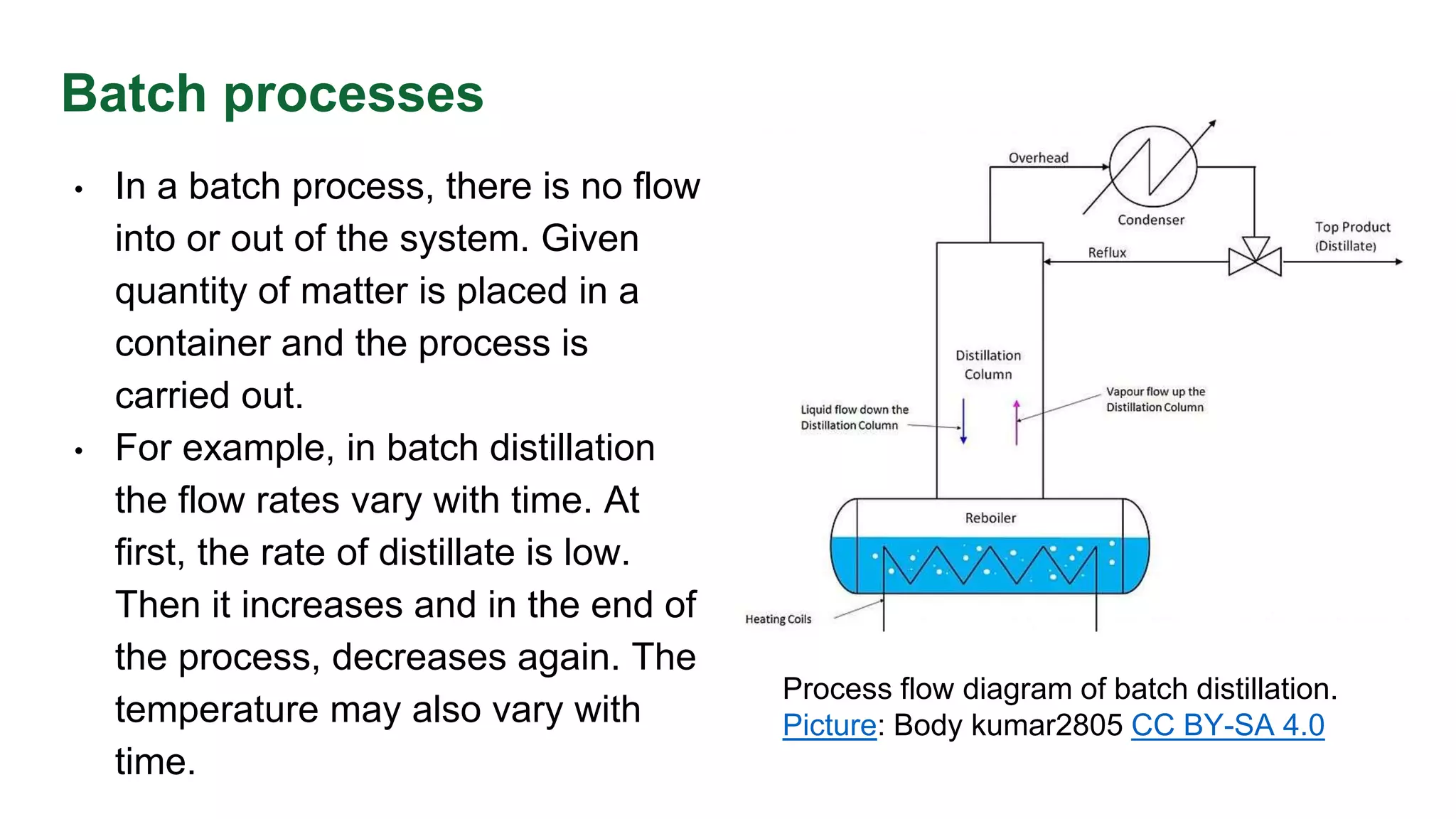
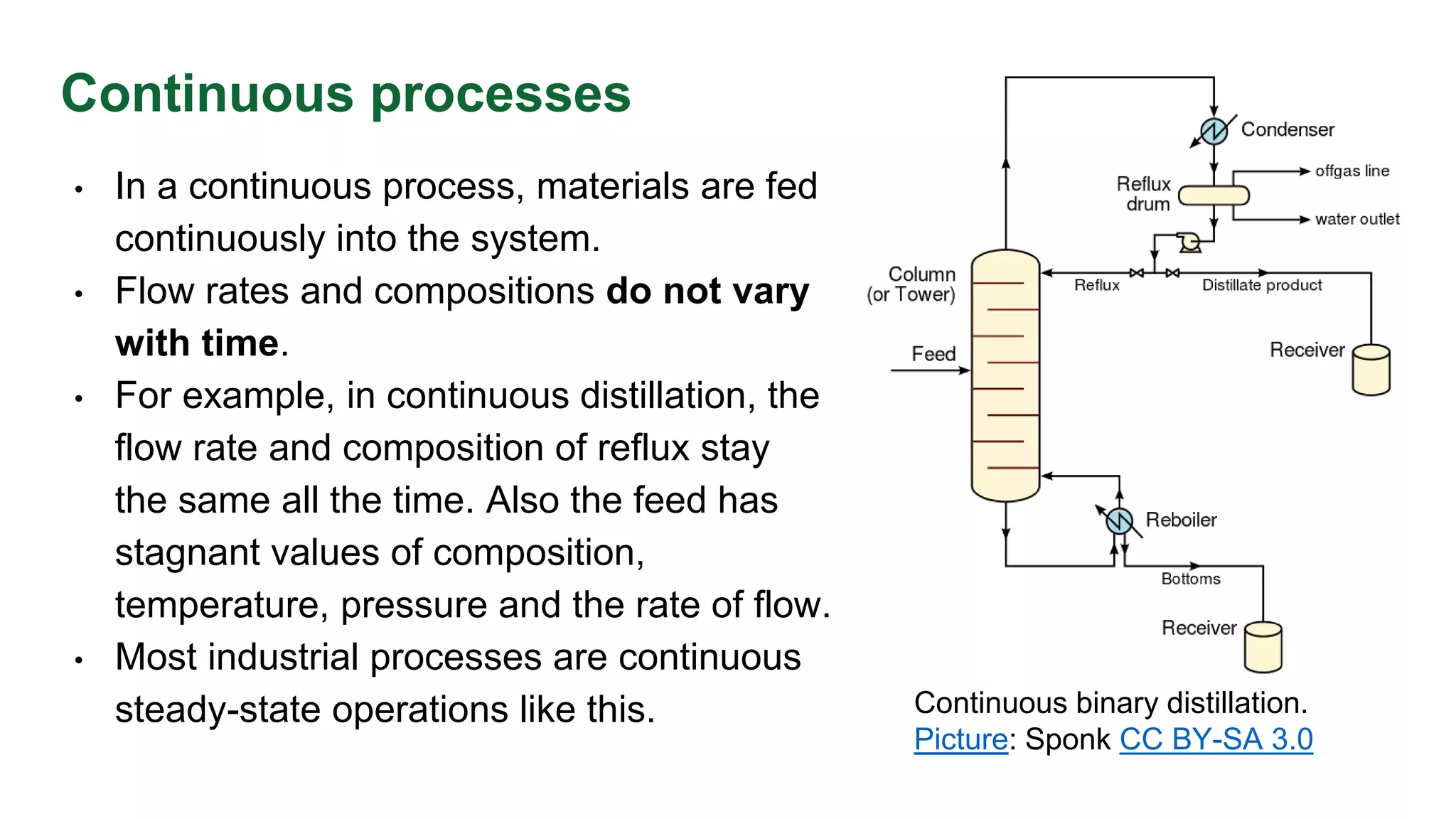
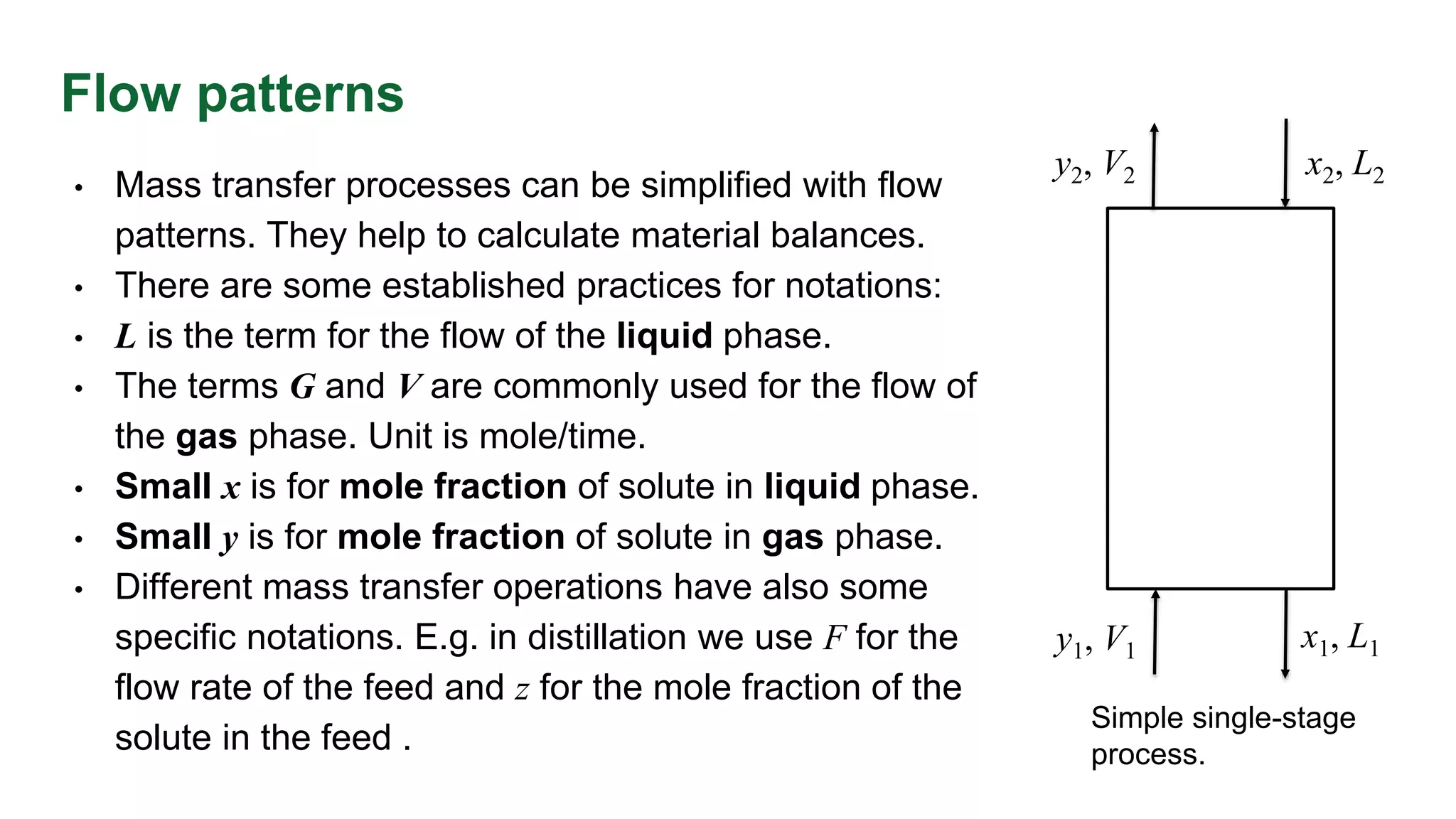
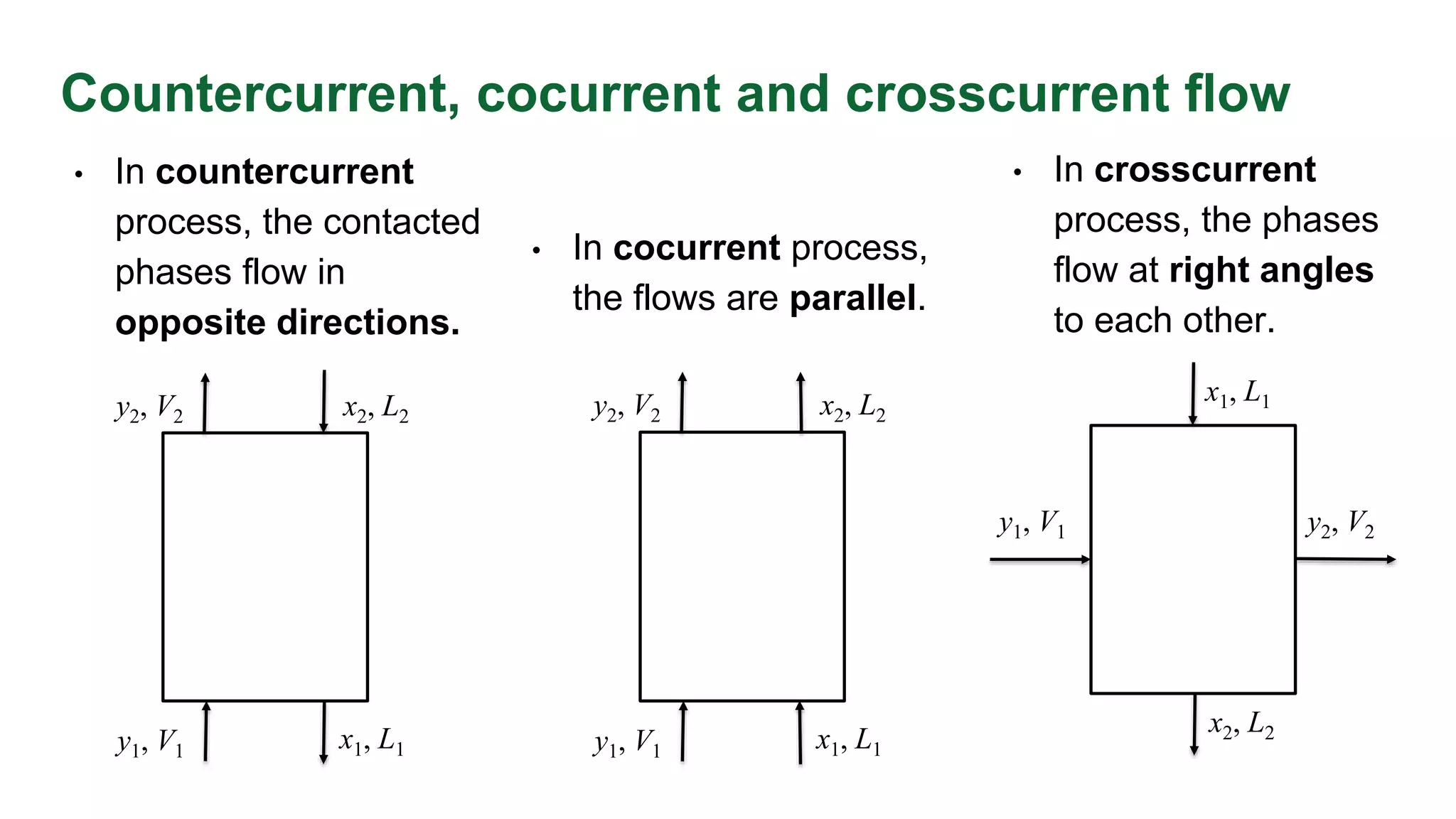
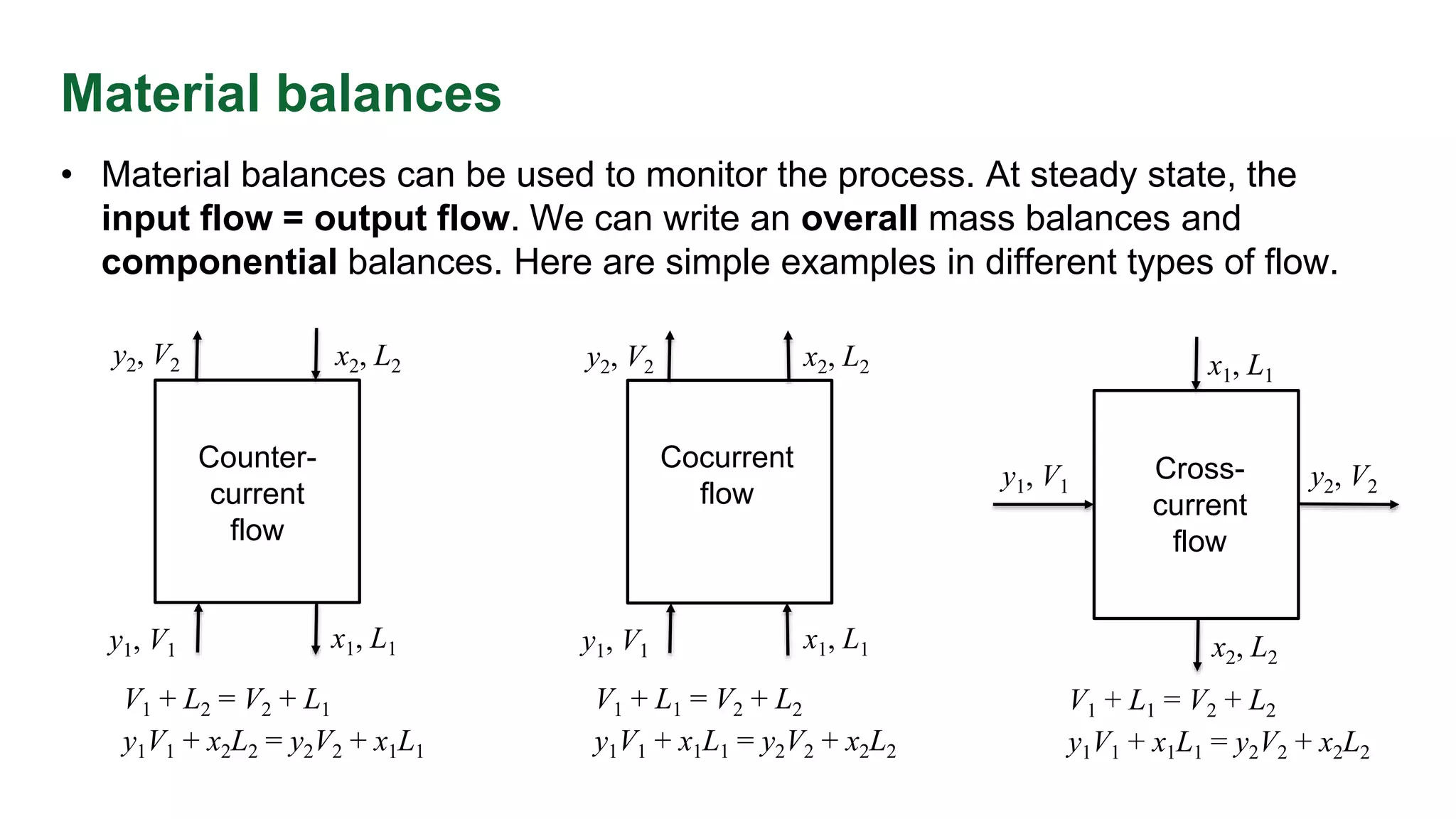
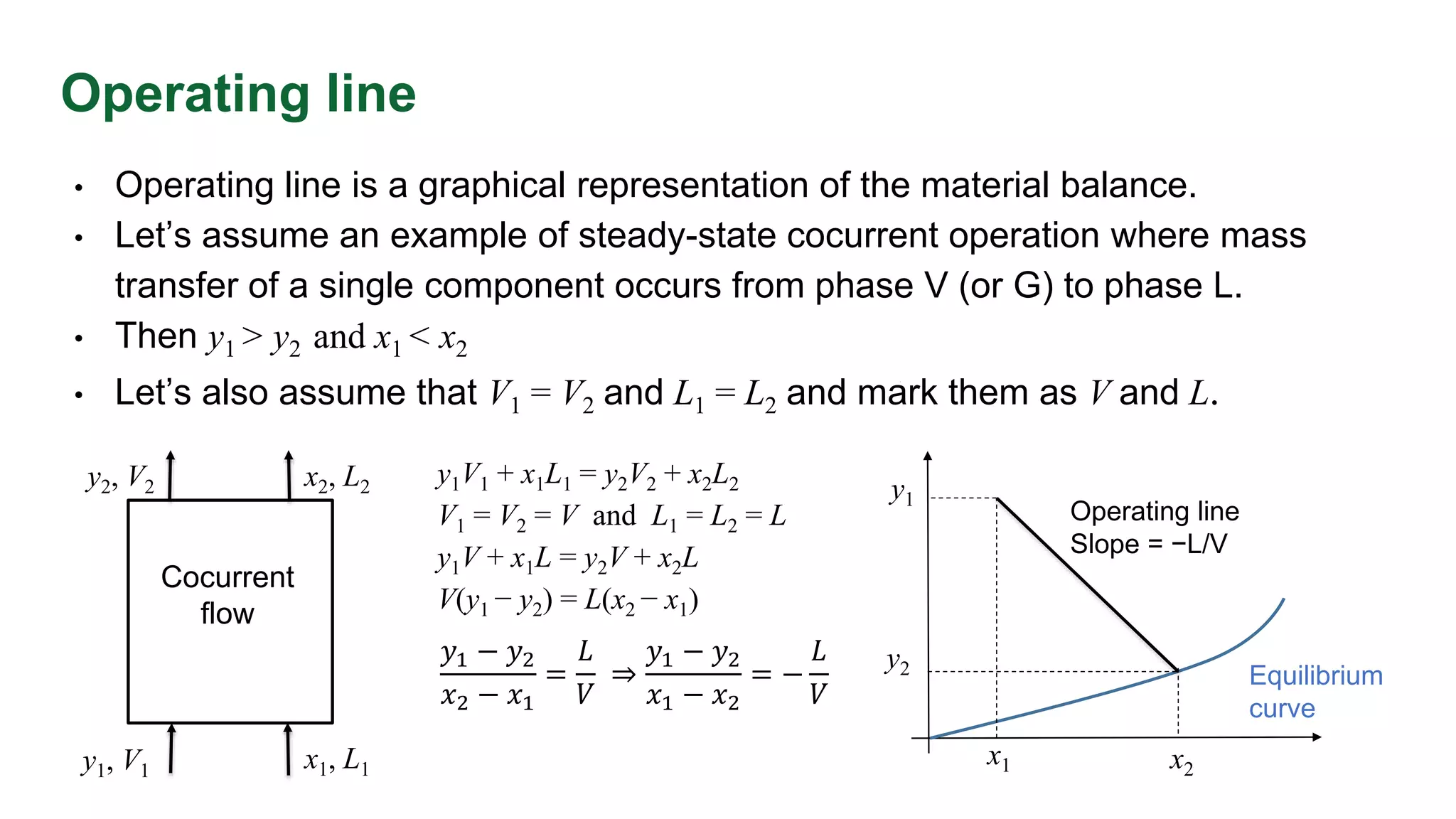
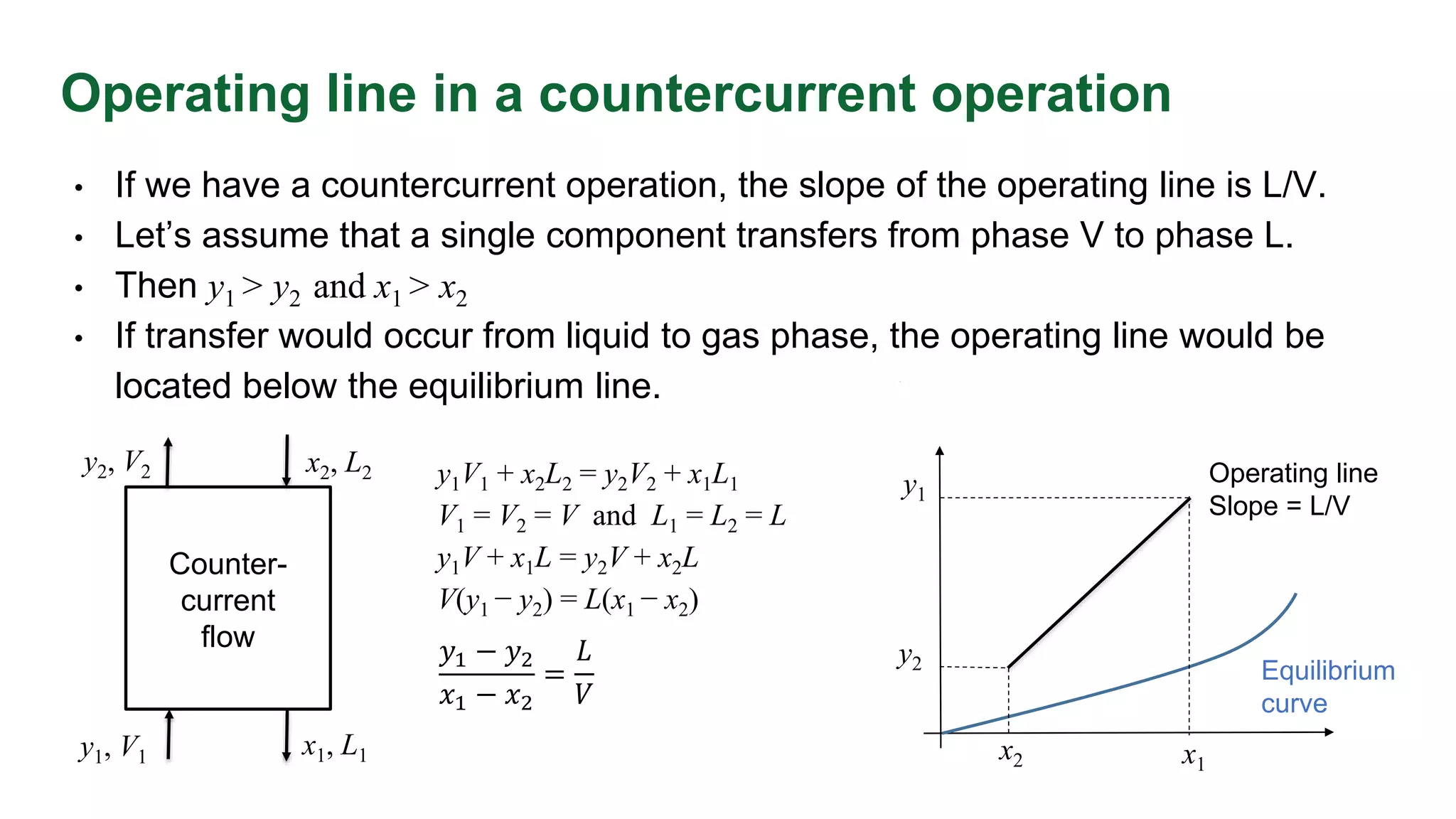
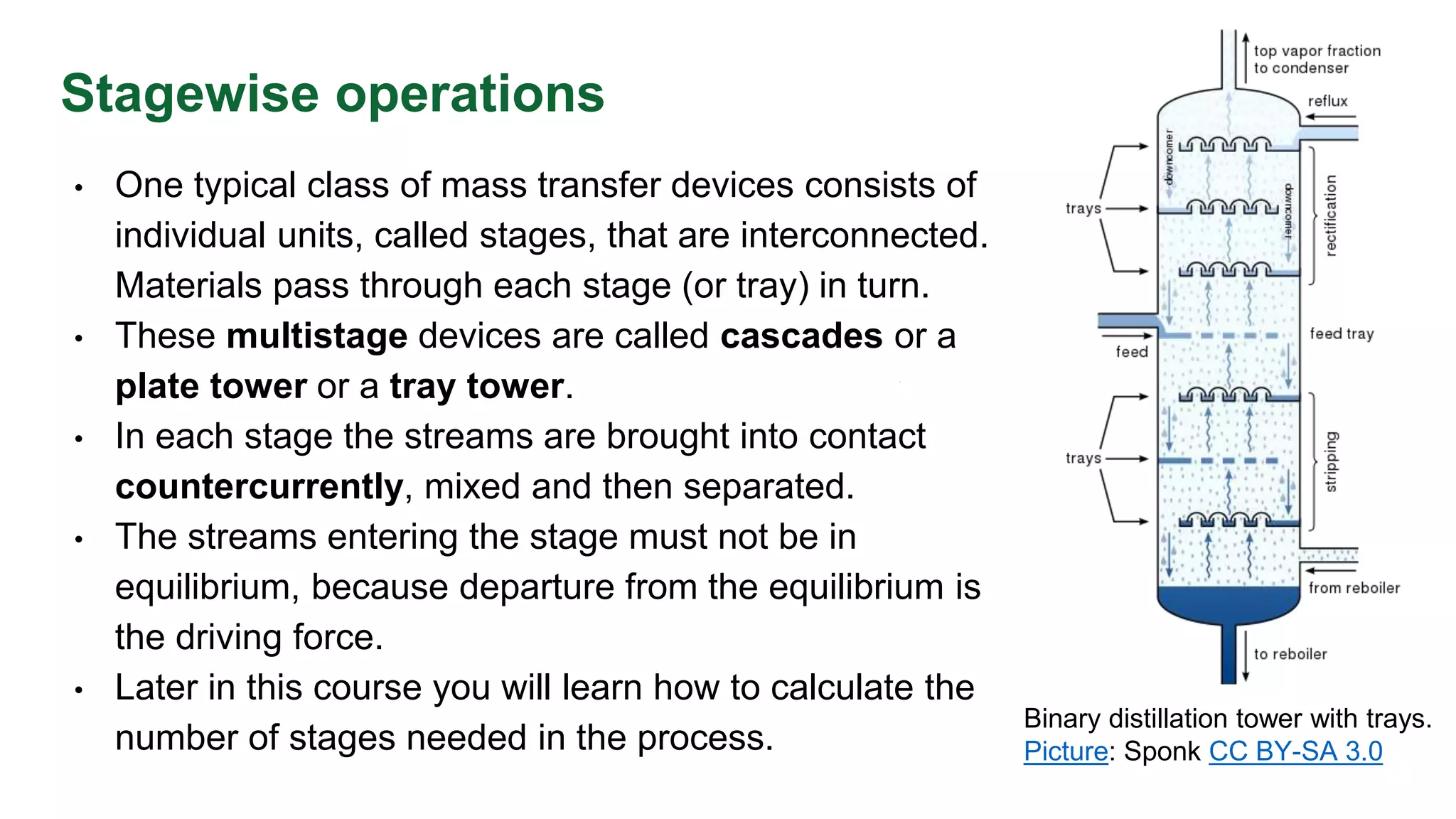
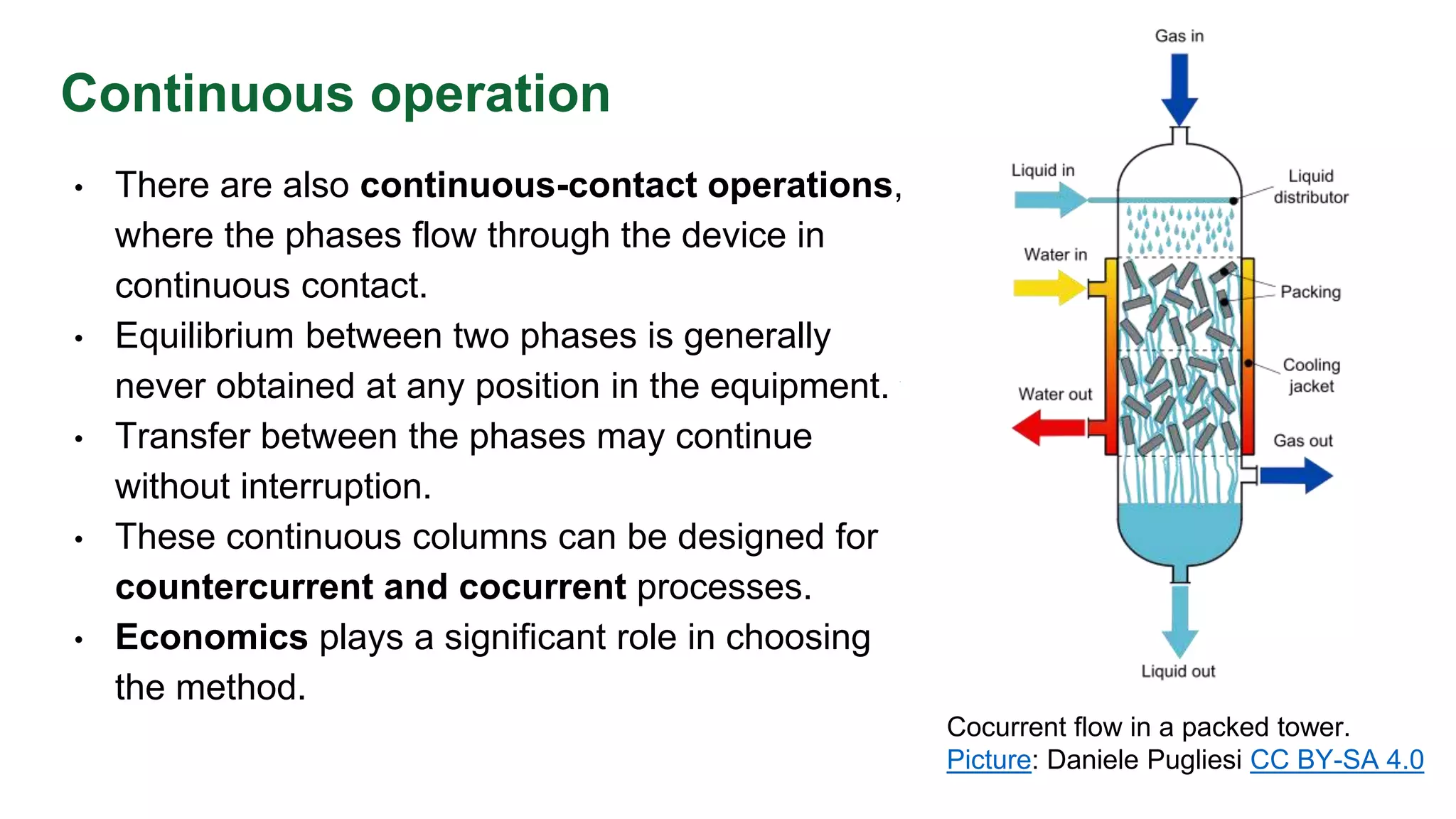
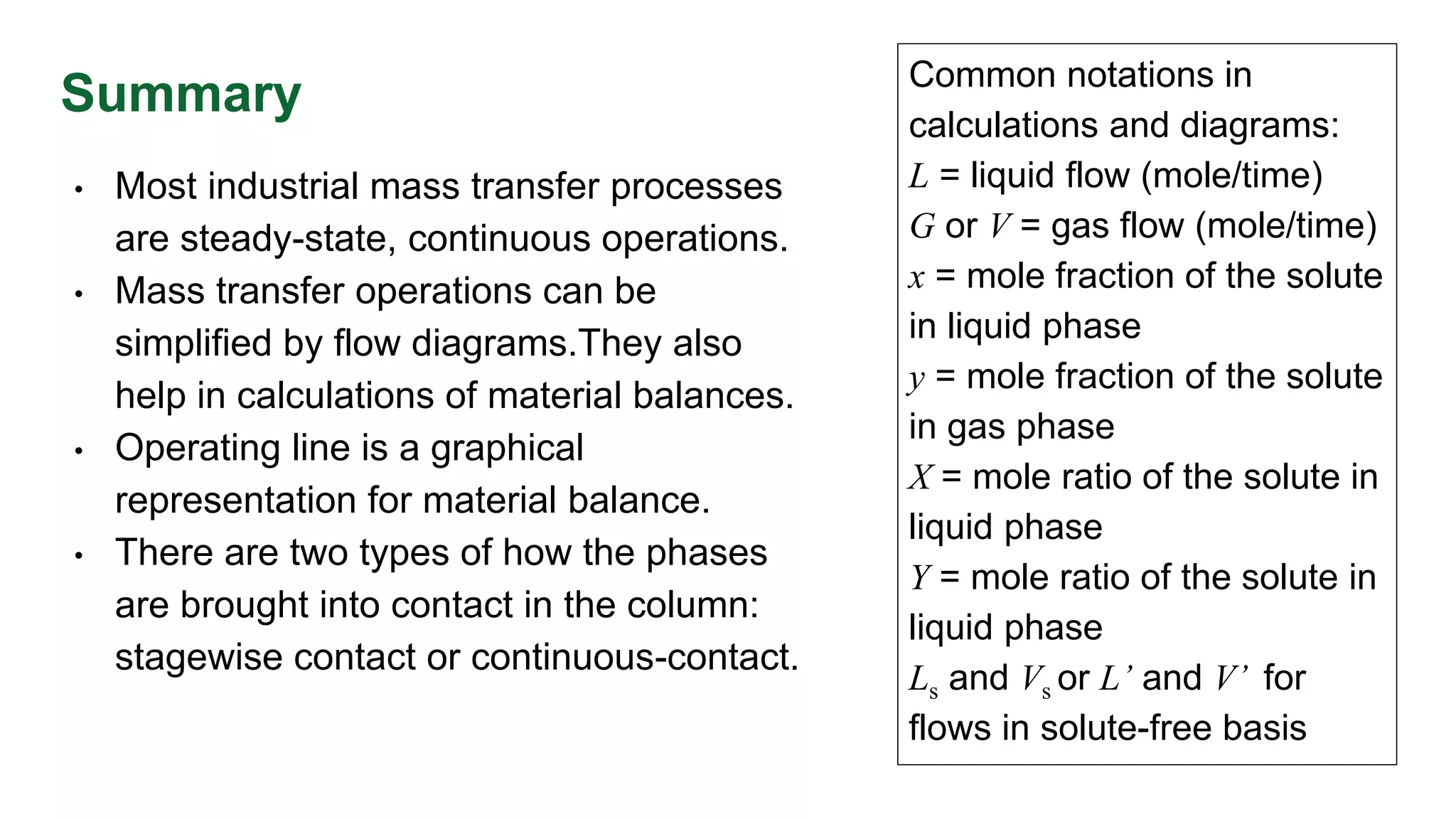
The document discusses mass transfer design principles, focusing on the differences between steady-state and unsteady-state operations, as well as batch and continuous processes. It covers material balances, flow patterns, and operating lines in mass transfer operations, highlighting the significance of flow diagrams in process calculations. The document emphasizes that most industrial mass transfer processes are continuous and steady-state operations, with specific notations for calculations.