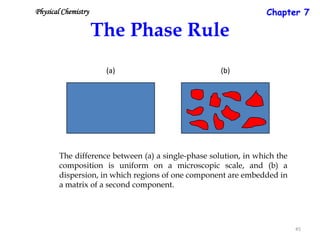
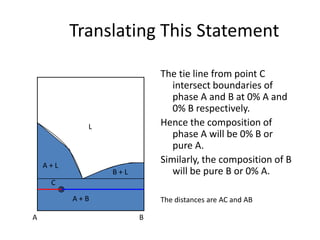
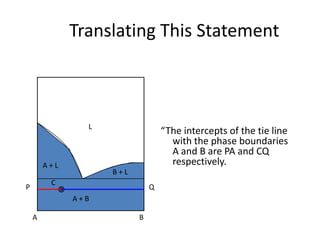
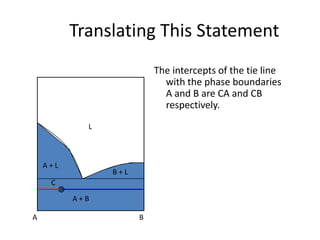
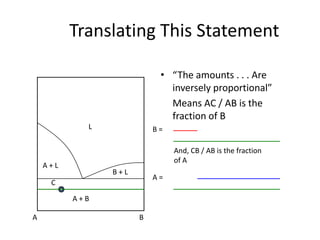
This document provides information about phase diagrams:
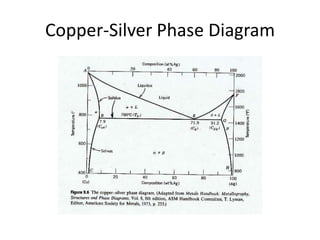
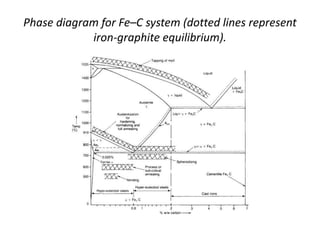
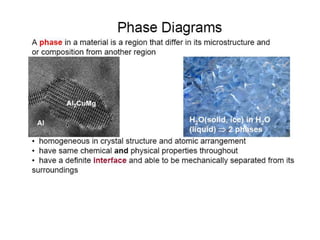
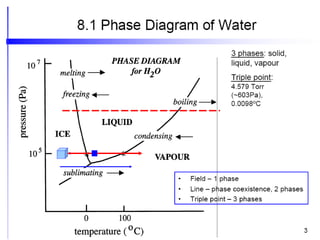
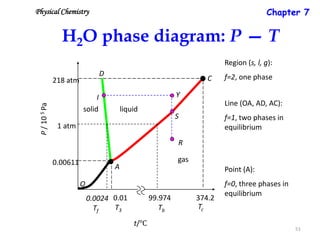
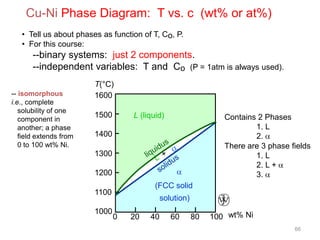
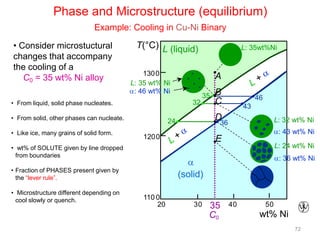
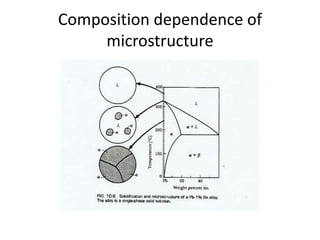
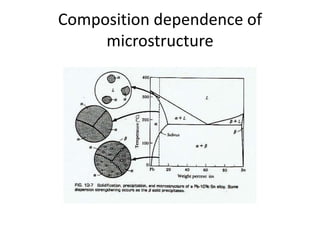
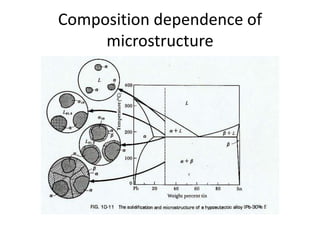
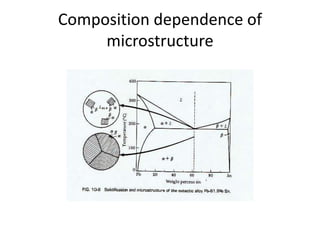
[1] Phase diagrams graphically show the phases present in a material system at different temperatures and compositions. They can indicate properties like the number, type, and amount of phases.
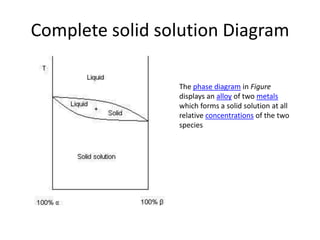
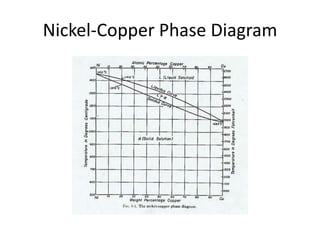
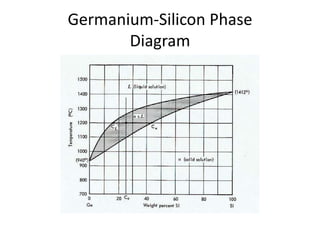
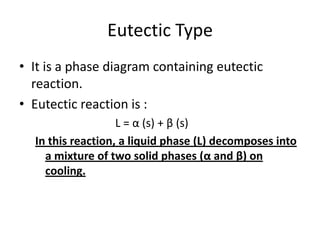
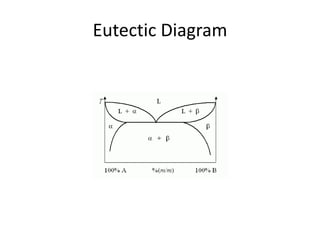
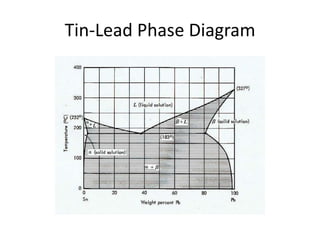
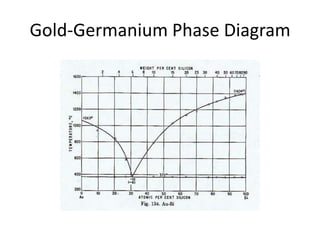
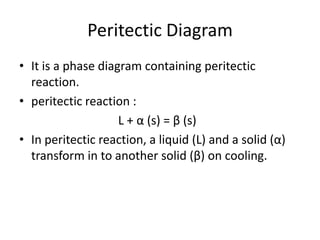
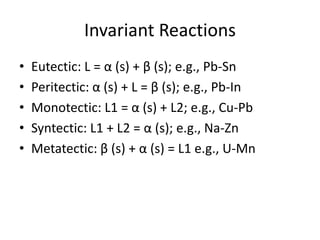
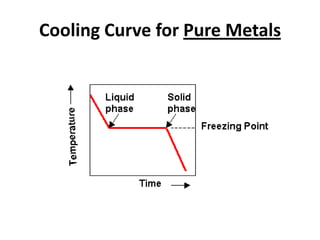
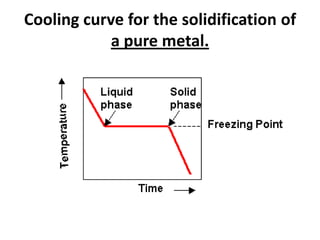
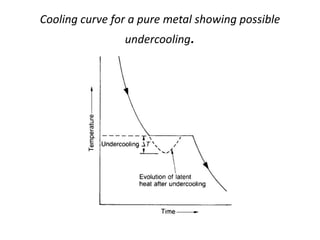
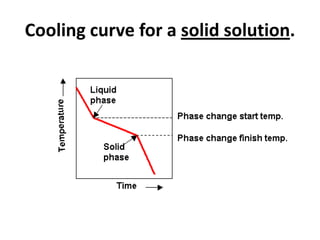
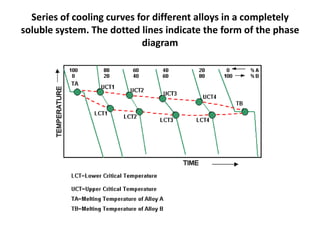
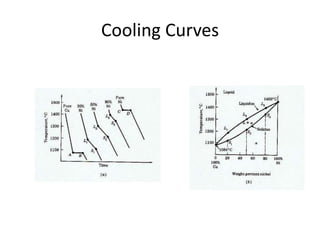
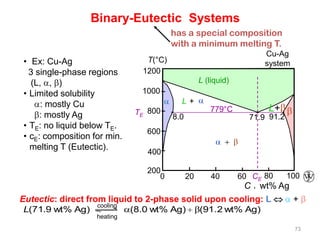
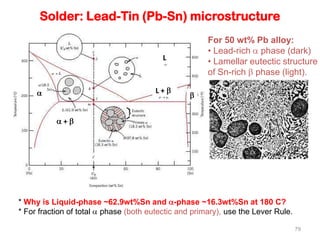
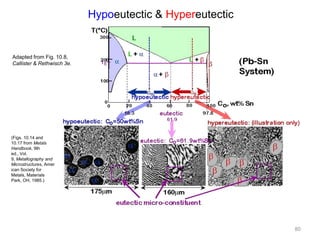
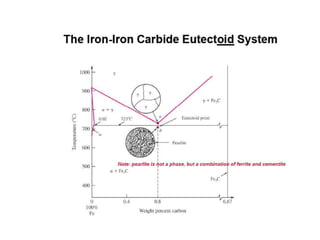
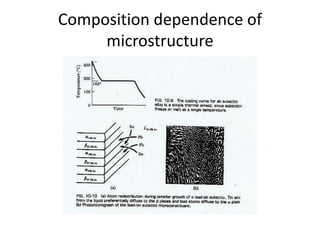
[2] There are several common types of phase diagrams including complete solid solution, eutectic, and peritectic diagrams. Cooling curves are also used to experimentally determine phase boundaries.
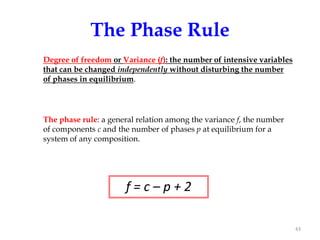
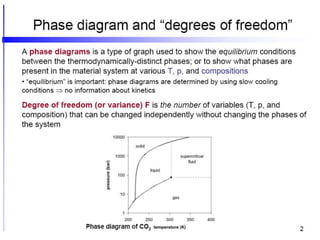
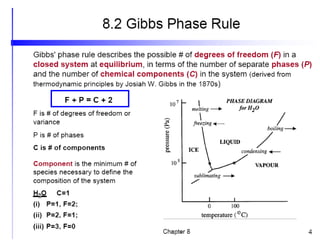
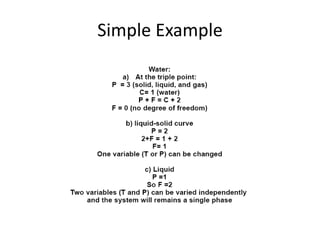
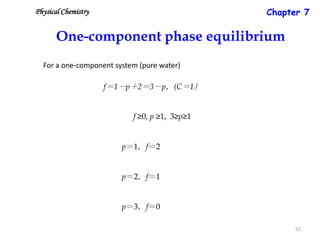
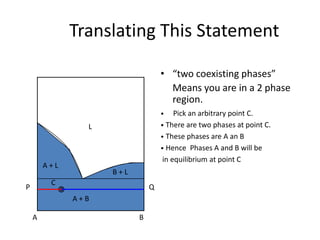
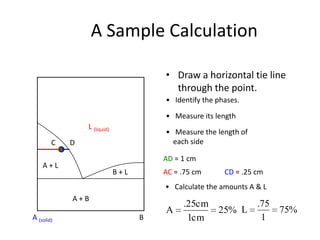
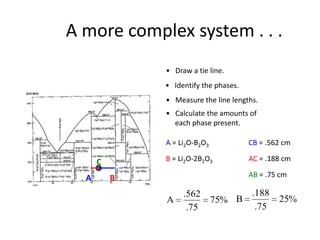
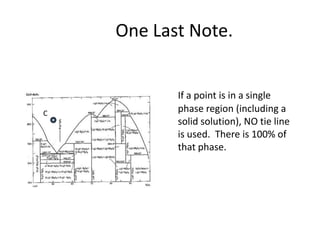
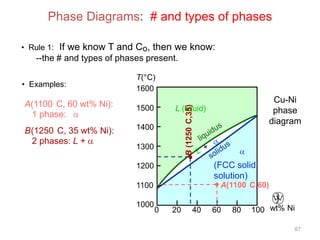
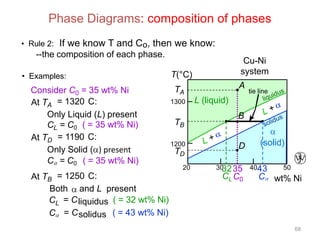
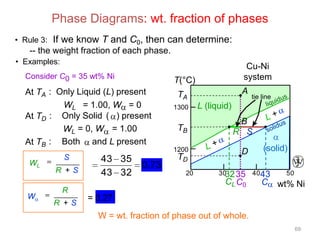
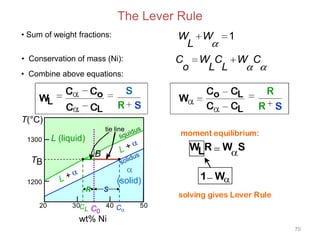
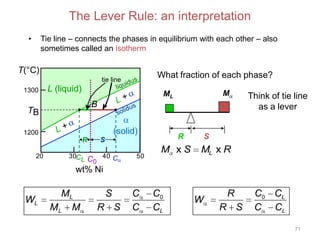
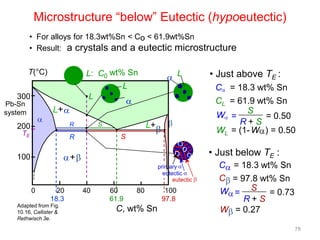
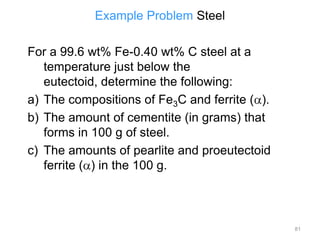
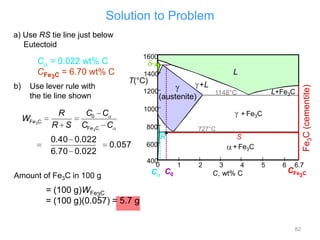
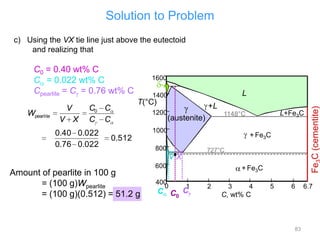
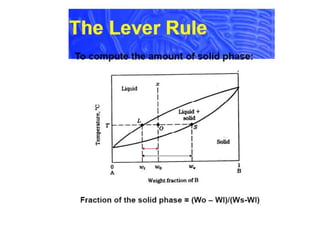
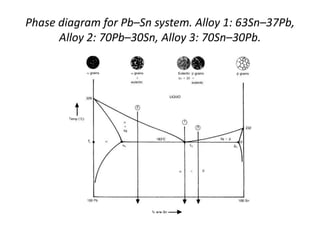
[3] The phase rule relates the number of phases, components, and degrees of freedom in a system. Lever rule calculations use tie lines on phase diagrams to determine the composition and relative amounts of coexisting phases.