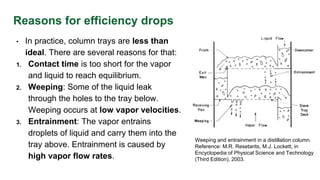
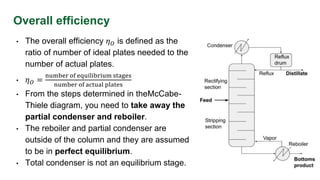
This document discusses the factors affecting plate efficiencies in distillation columns, particularly the concepts of overall, Murphree, and local efficiencies. It outlines how various interferences, such as weeping and entrainment, impact efficiency, and details the calculations involved in determining the number of actual trays required based on ideal trays. The text is supported by references and empirical correlations relevant to distillation processes.