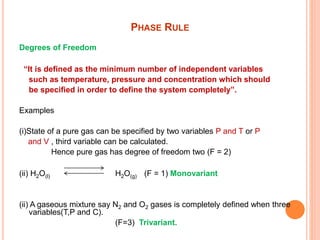
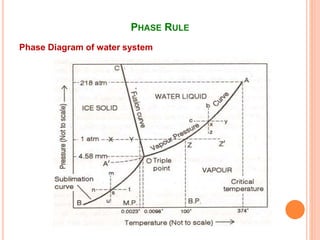
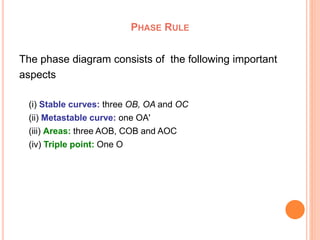
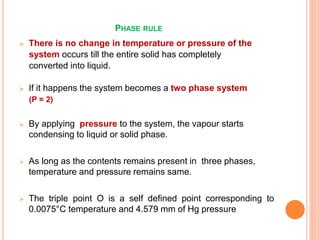
The document discusses the phase rule as it applies to water systems. It provides an overview of Gibbs phase rule and its key terms like phases, components, and degrees of freedom. It then presents the phase diagram for a one-component water system. The phase diagram shows the three stable phases of ice, liquid water, and water vapor that exist at different temperature and pressure conditions. It also identifies the triple point where the three phases coexist in equilibrium and discusses the different areas and curves on the phase diagram related to phase boundaries and transitions.