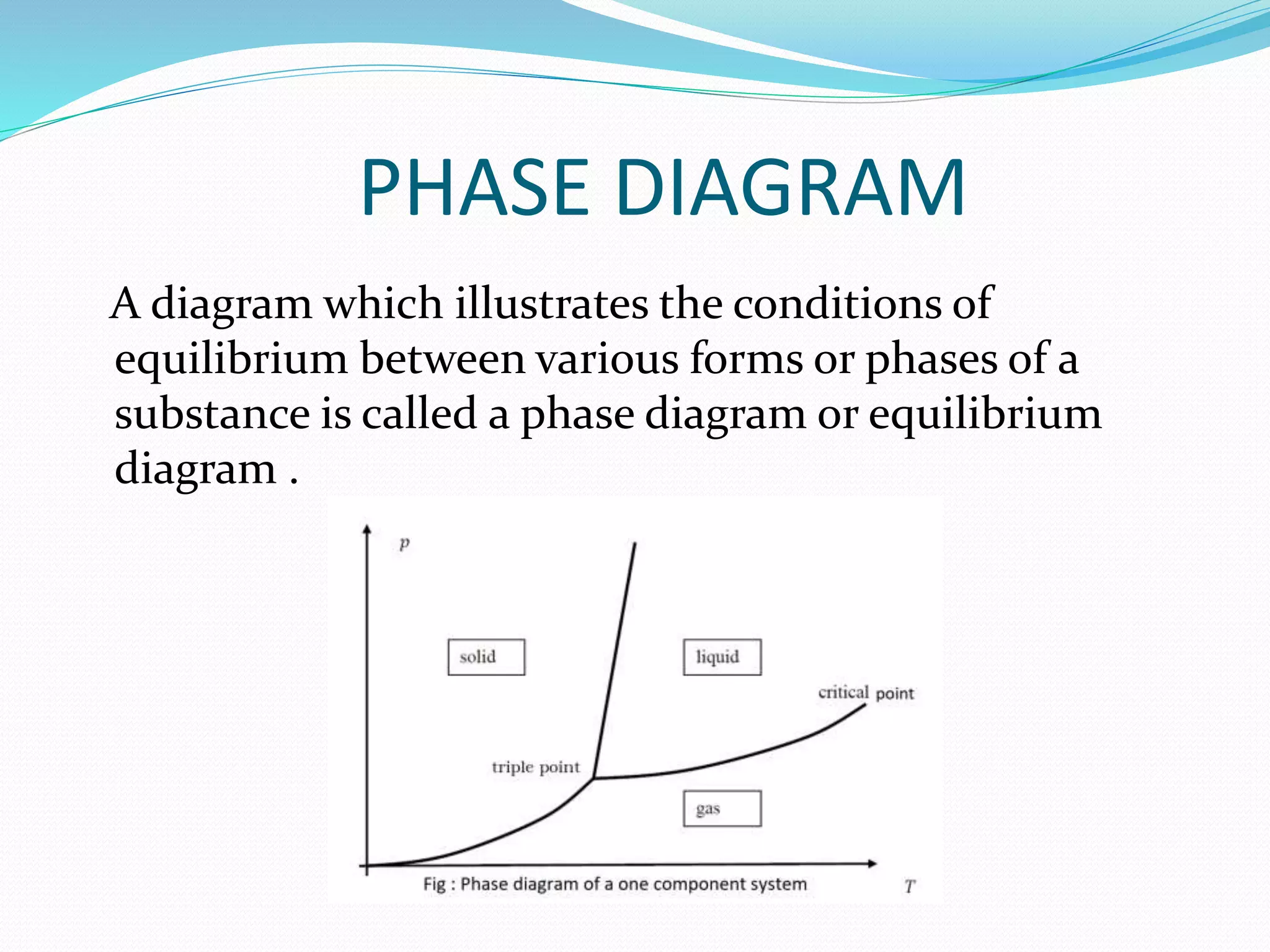
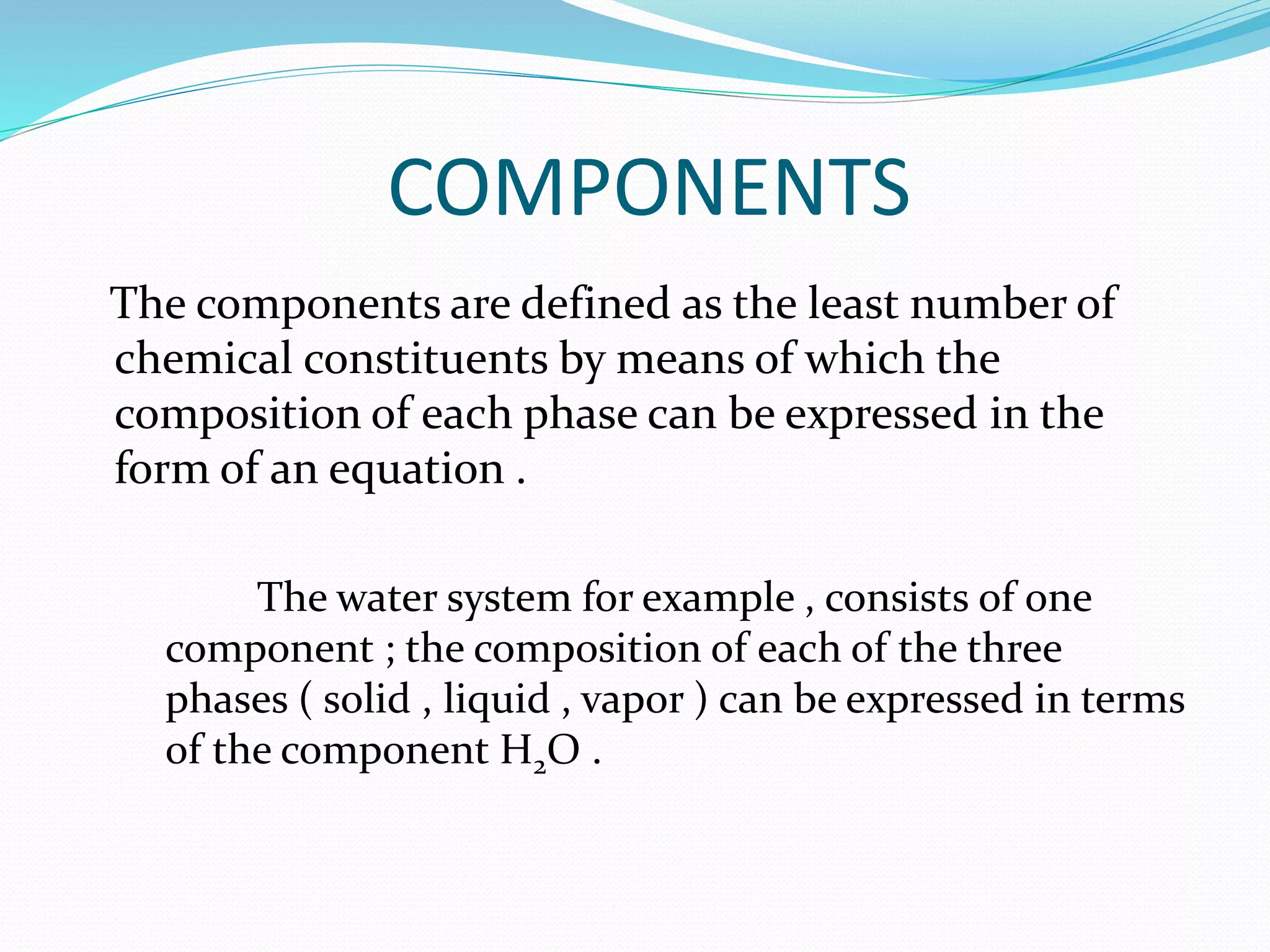
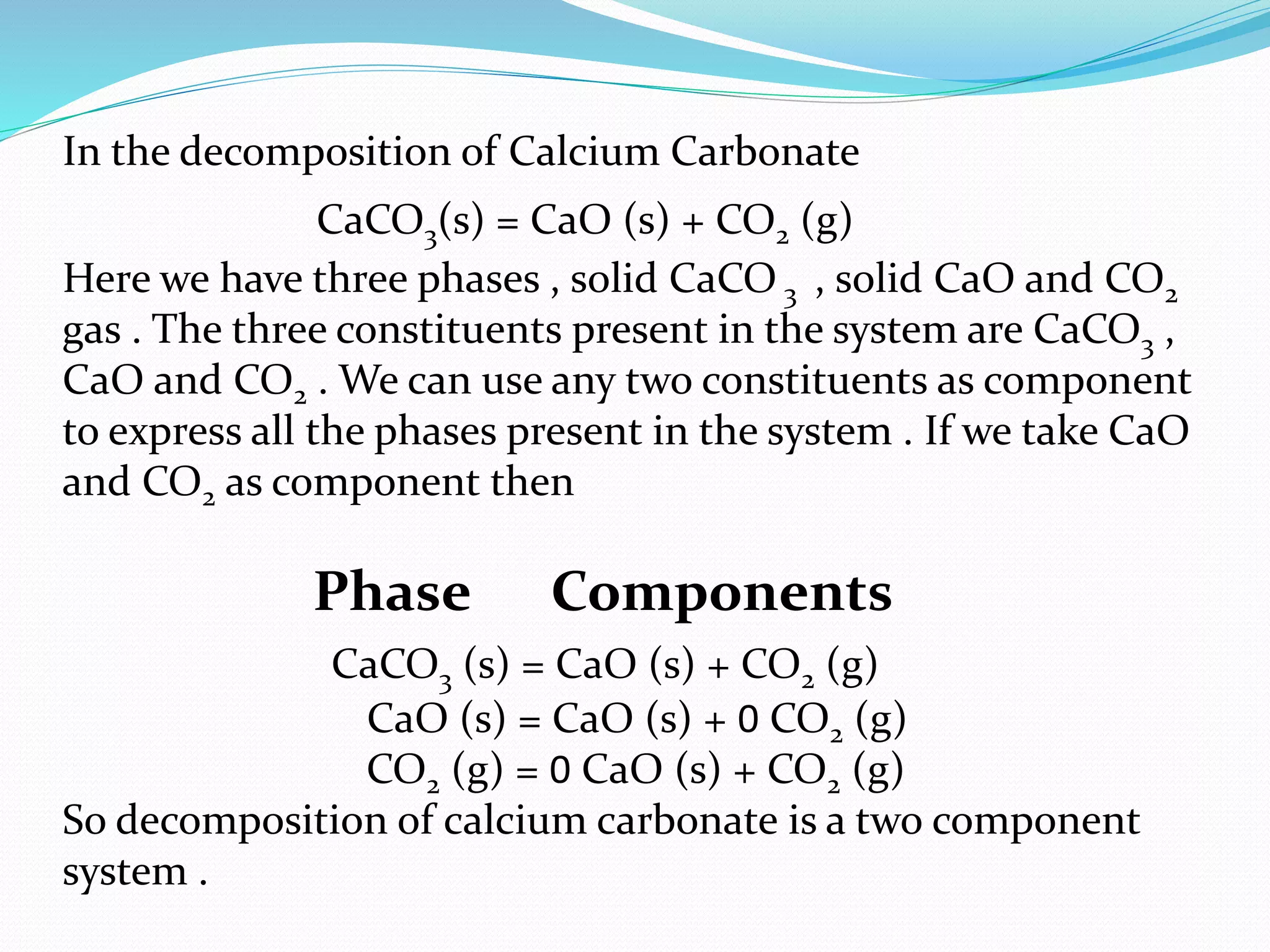
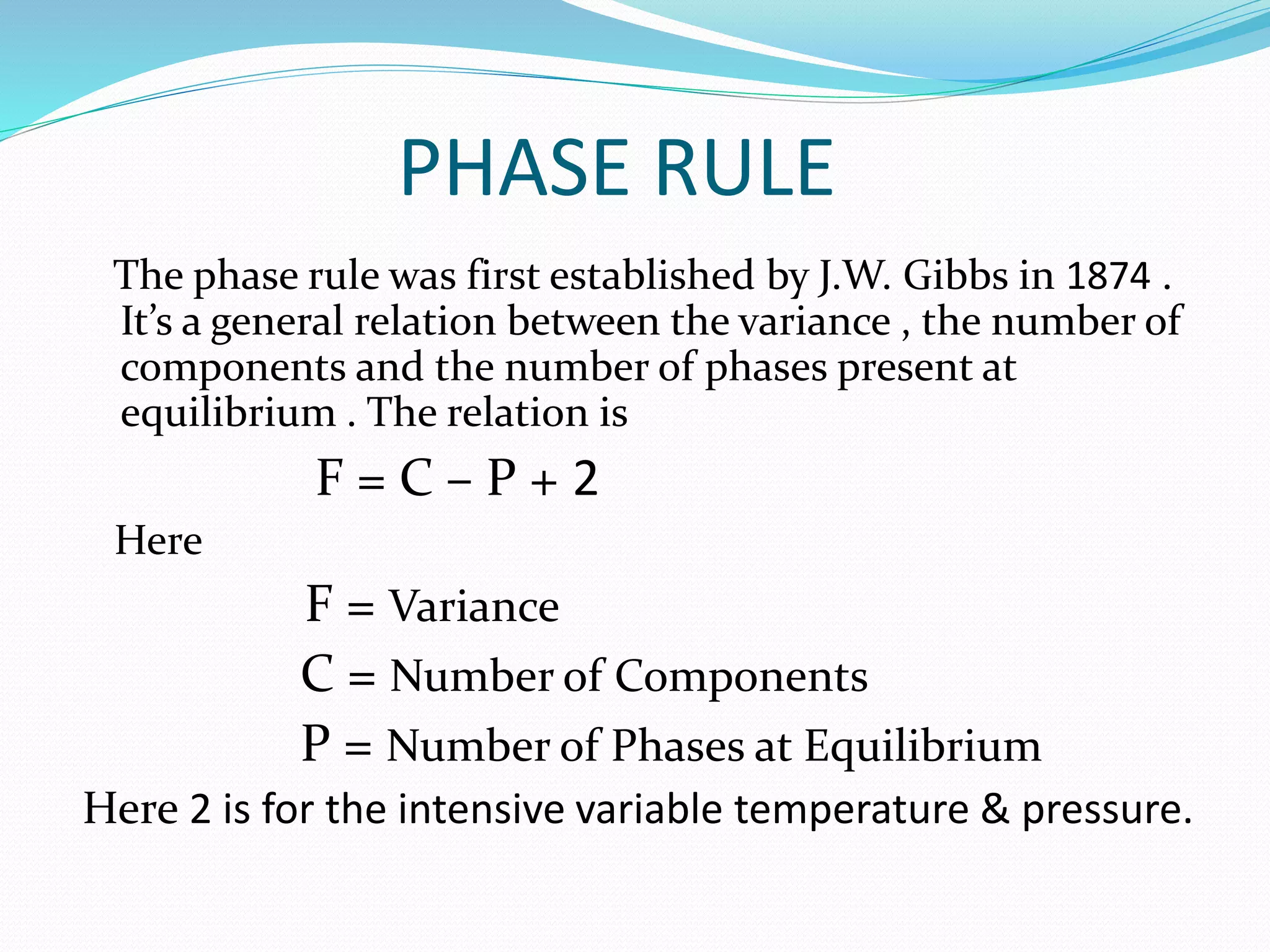
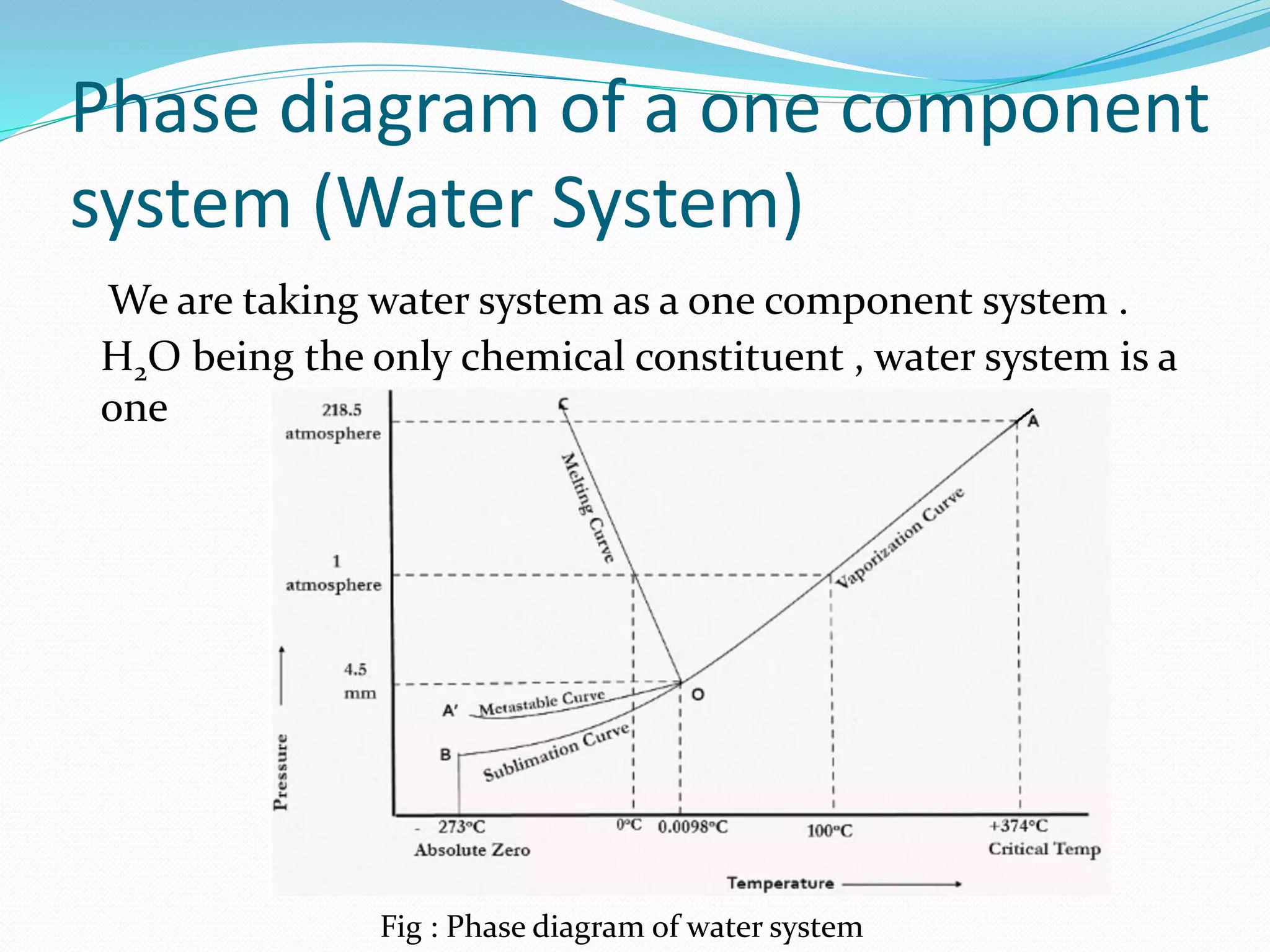
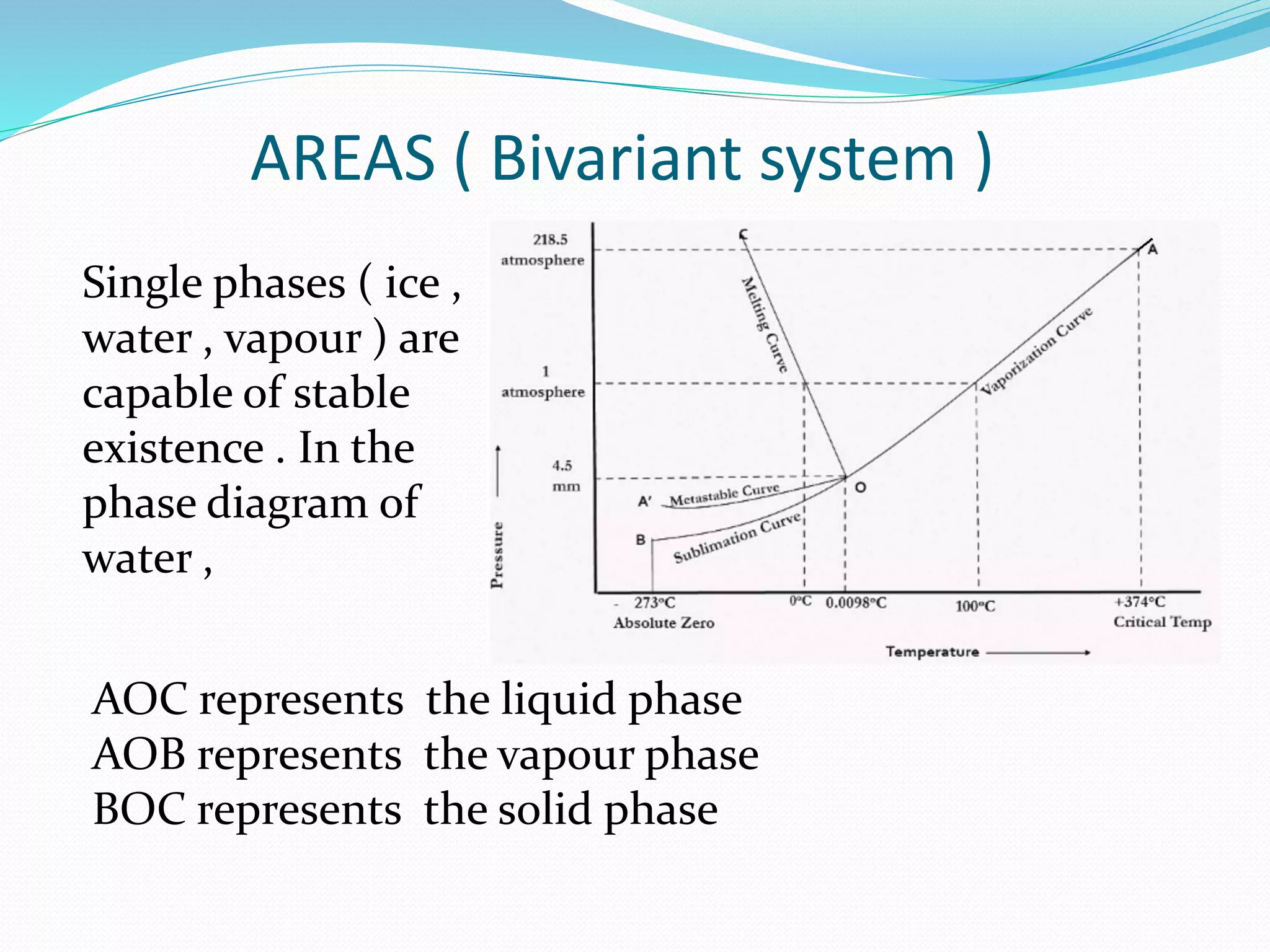
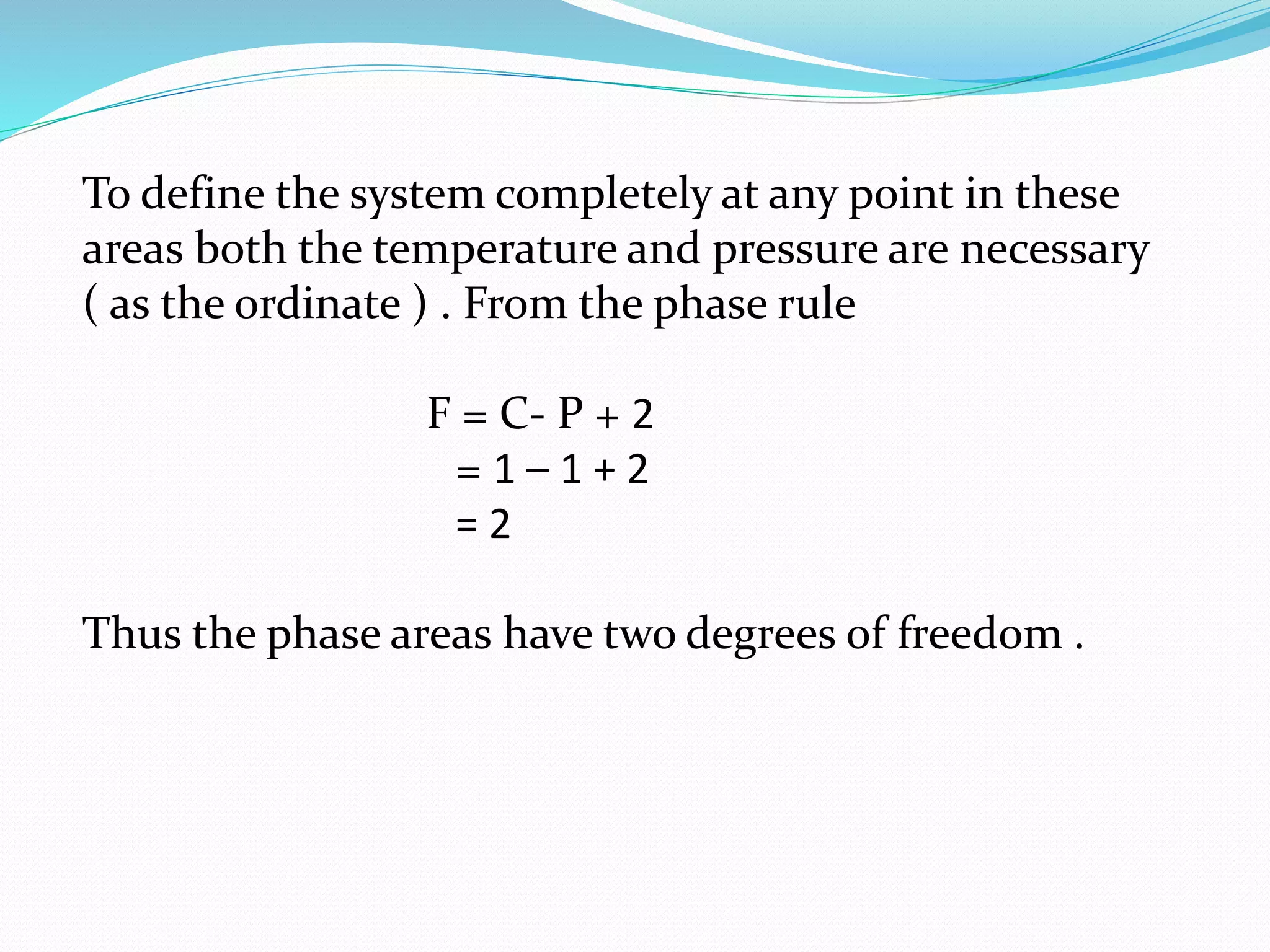
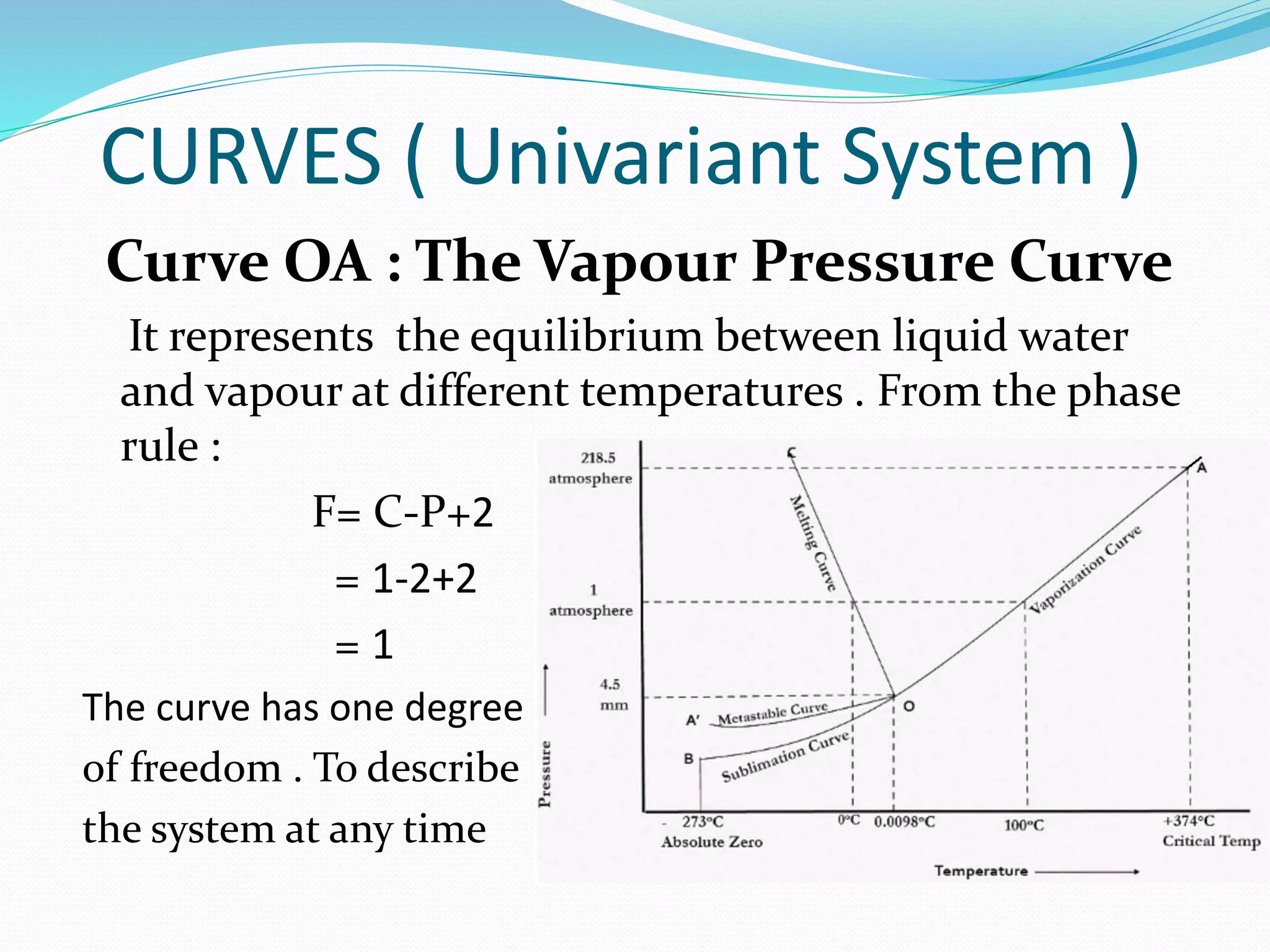
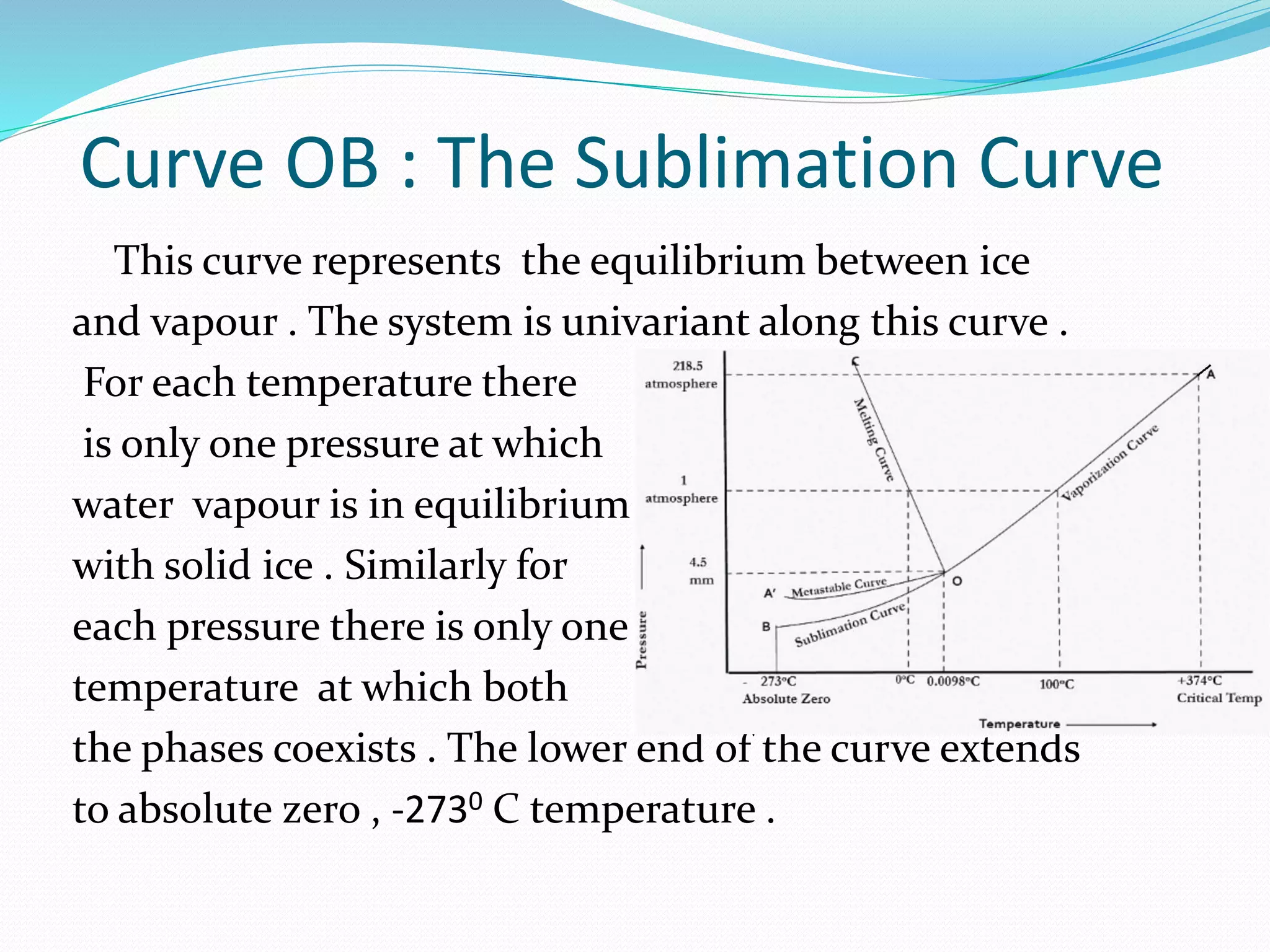
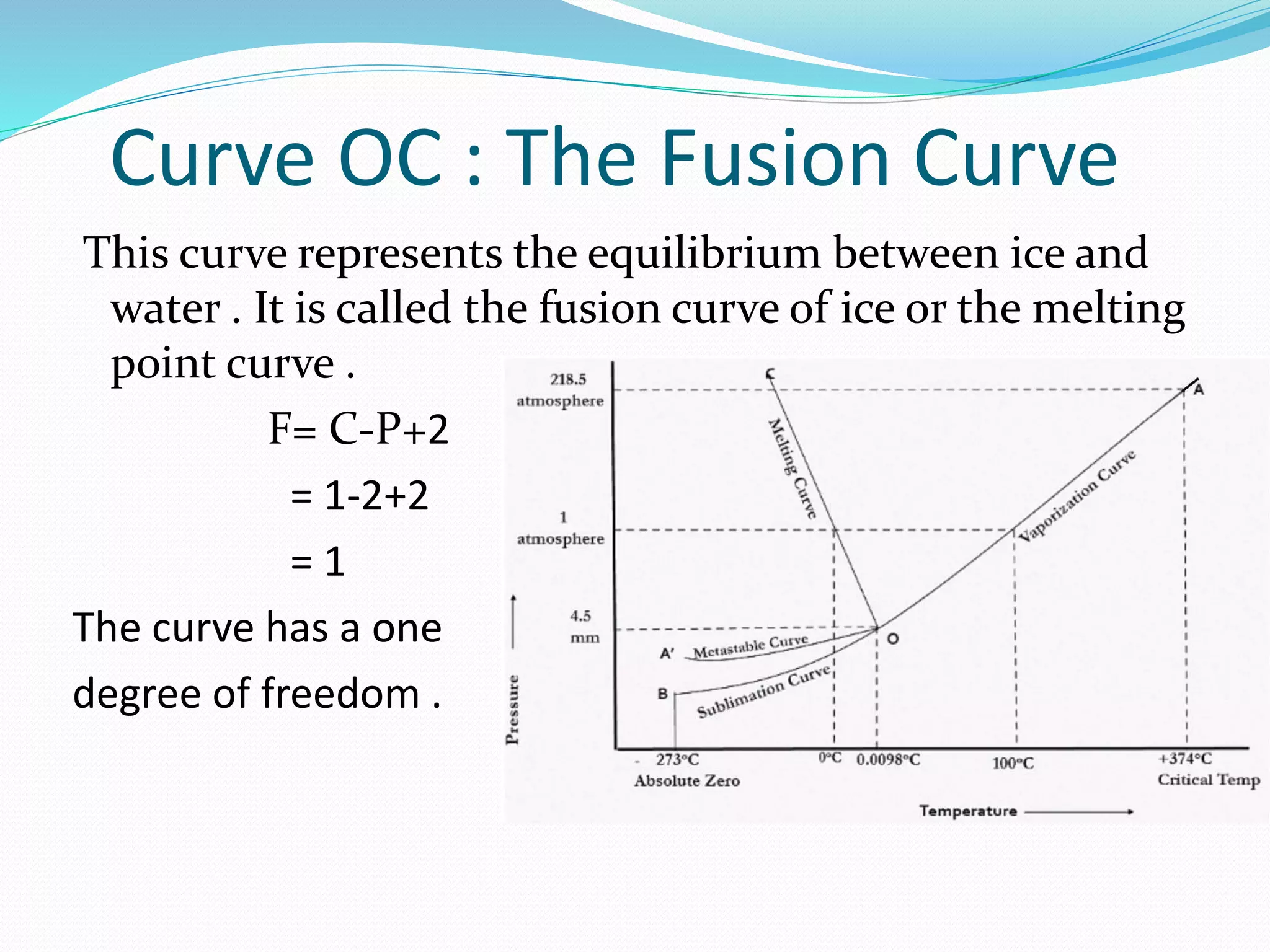
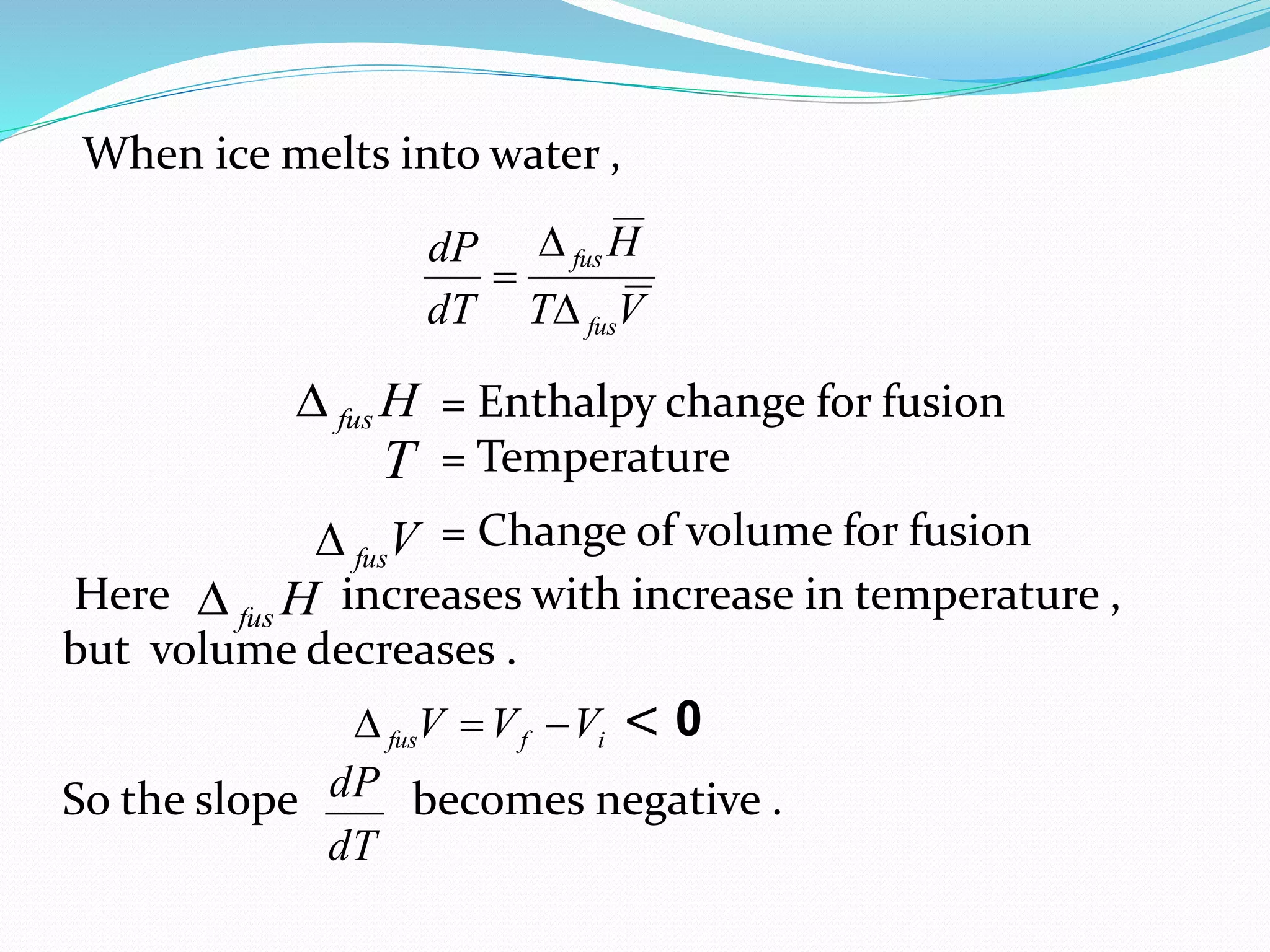
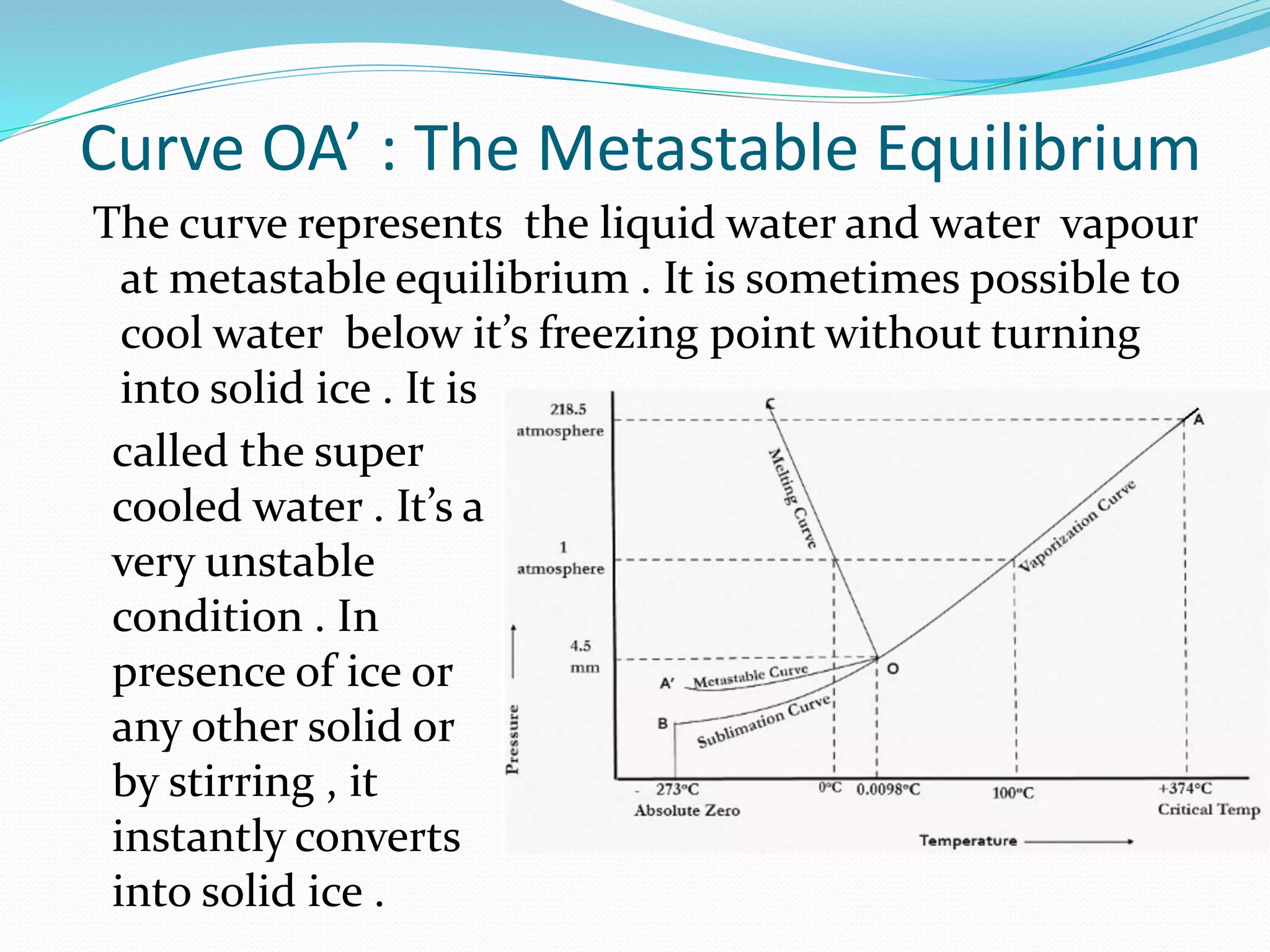
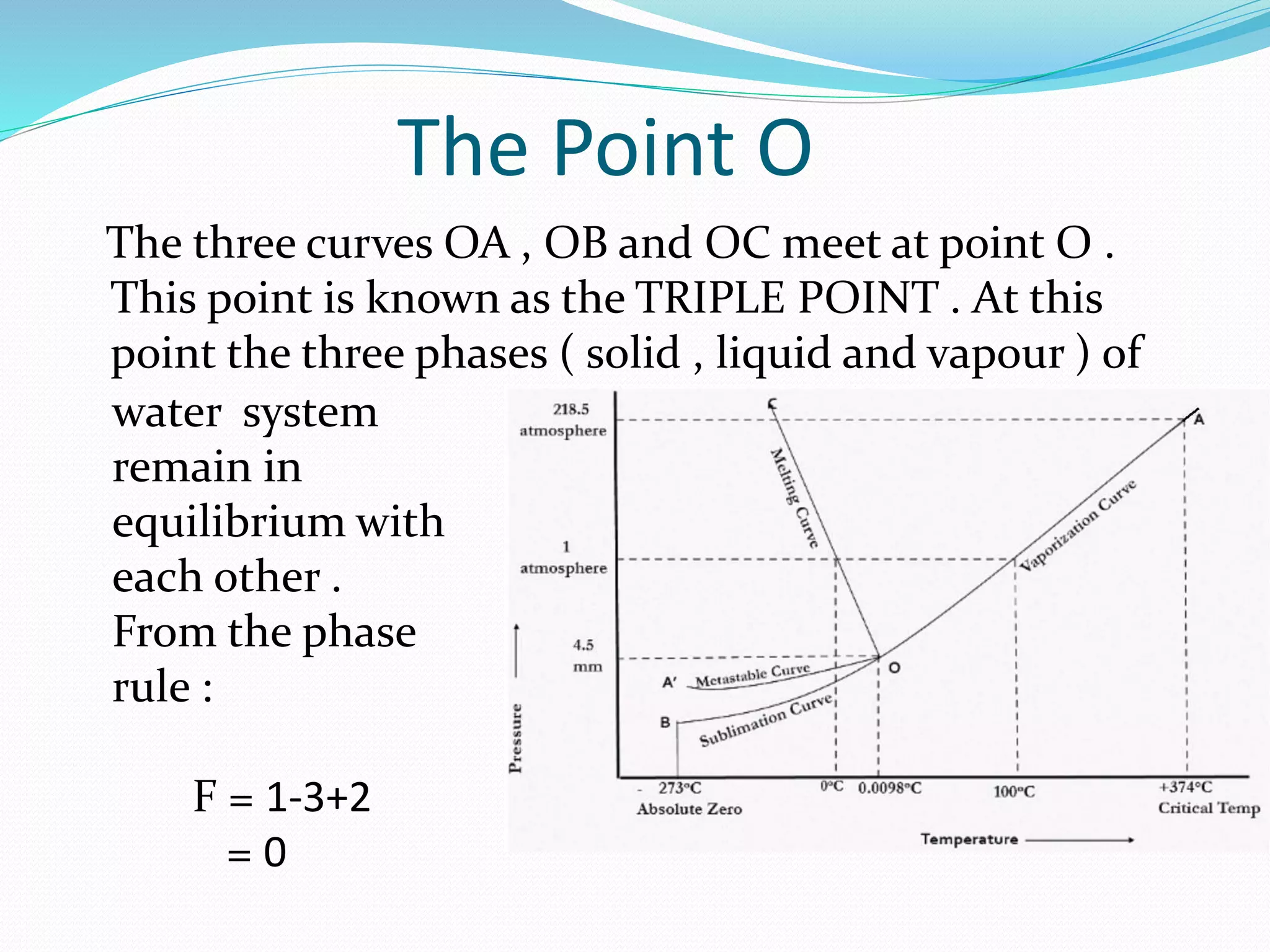
This document discusses phase diagrams and the phase rule through the example of a one-component water system. It defines key terms like phase, component, degree of freedom, and phase rule. It then explains the different areas and curves in the phase diagram of water, including that the liquid-vapor, ice-vapor, and ice-liquid curves represent univariant systems while the areas represent bivariant systems. It also notes the unique properties of the ice-liquid curve and the triple point where ice, liquid water, and vapor coexist in equilibrium.