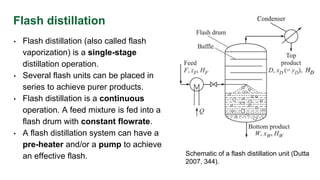
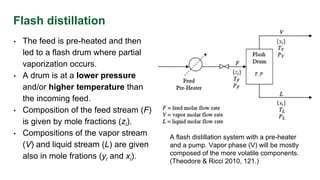
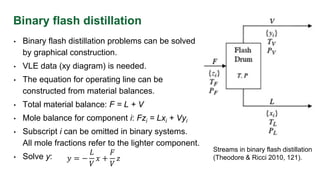
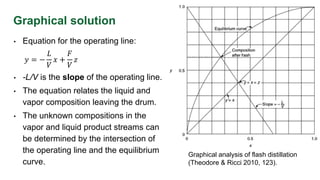
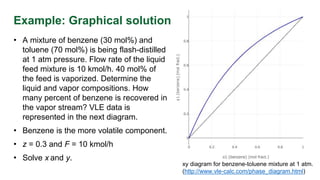
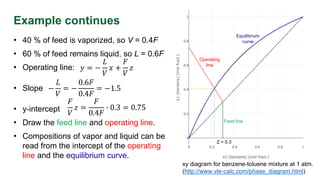
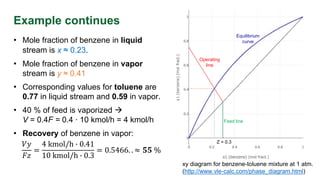
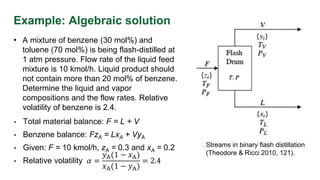
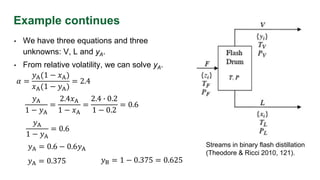
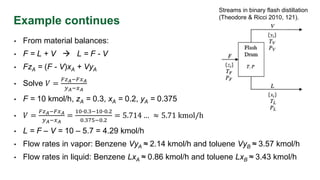
The document discusses flash distillation, a continuous distillation process involving partial vaporization, typically of feed mixtures. It covers both binary and multicomponent flash distillation, including calculations for vapor and liquid compositions and recovery rates using material balances and graphical methods. Additionally, it references key literature and educational videos for further understanding of the process.