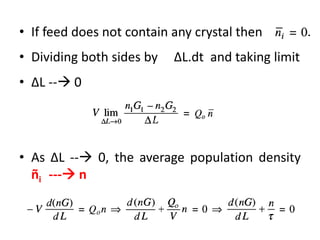
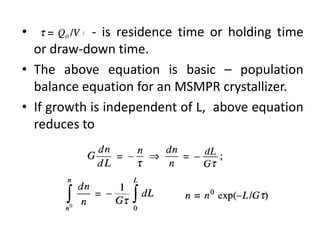
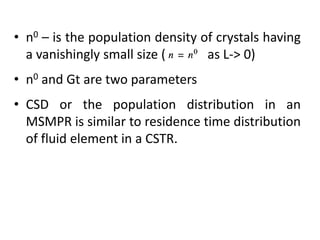
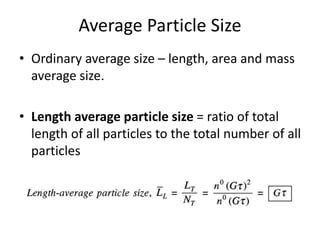
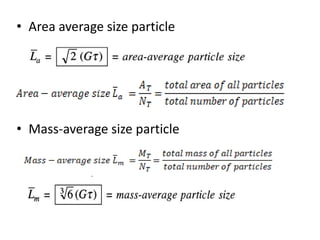
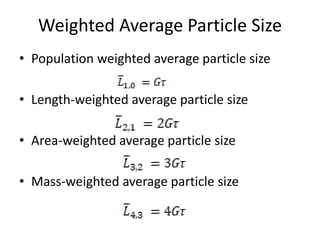
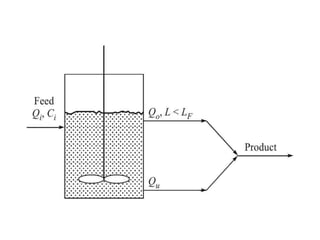
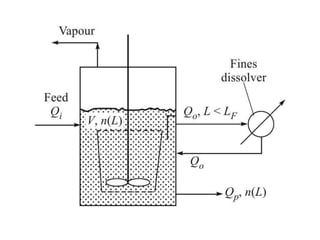
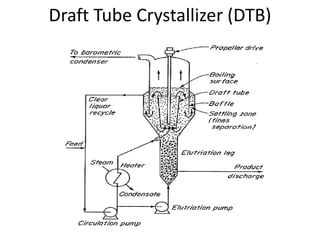
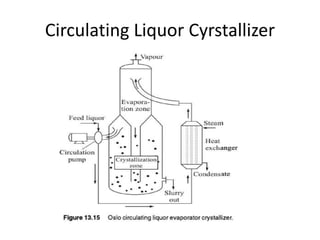
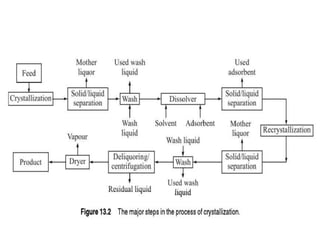
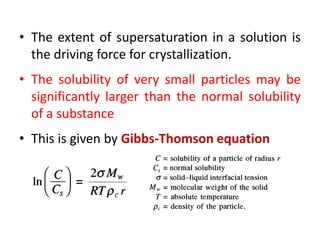
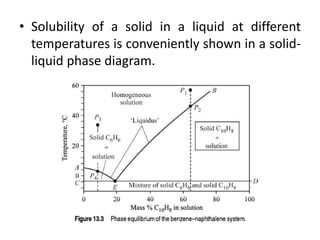
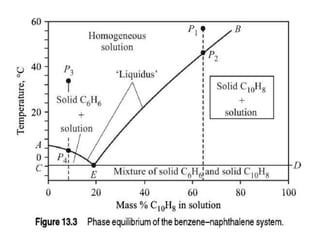
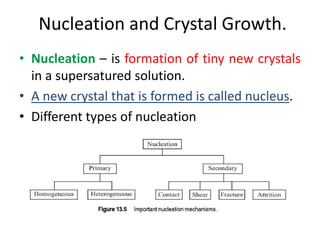
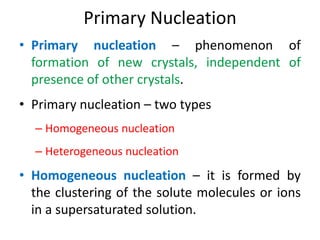
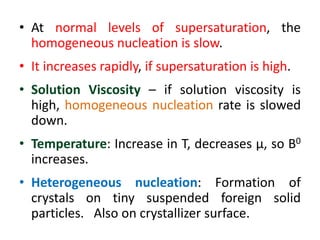
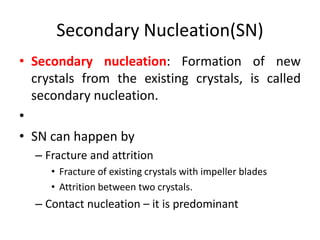
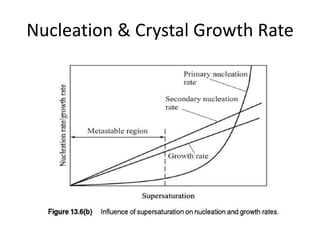
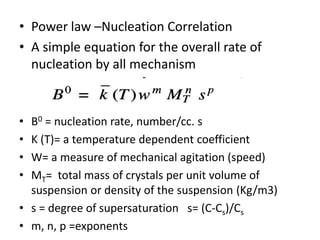
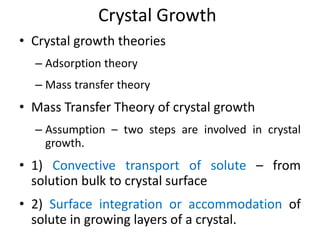
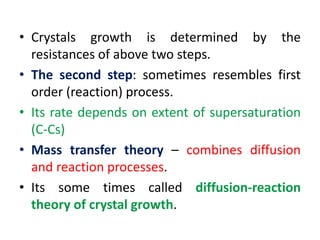
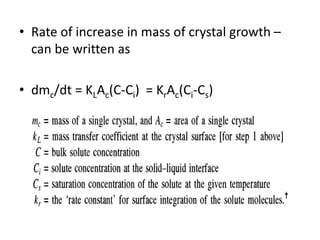
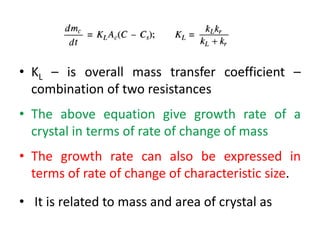
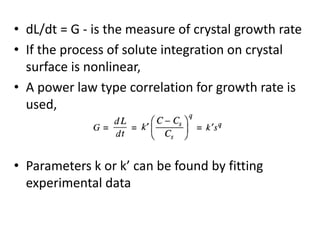
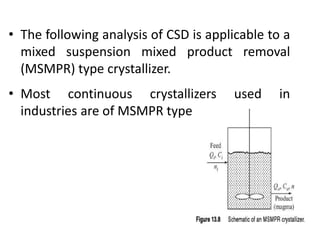
This document provides an overview of crystallization processes. It discusses how crystals form from solutions or melts via nucleation and growth. Primary and secondary nucleation are described. Mass transfer and population balance theories are used to model crystal growth rates and size distributions. The document outlines how continuous crystallizers like MSMPR systems operate and how residence time affects crystal size distribution. Methods for controlling crystal size like double draw-off, fines removal, and classified product removal are also summarized.















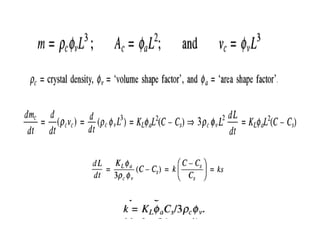


![• Population density for the size L1 is n1
• Population density for the size L2 is n2
• The average population density in the size
range L1 to L2 be - ñ
• Over a small time, dt
• Some crystals little small than L1, would grow
and enter the size range [L1, L2]](https://image.slidesharecdn.com/crystalization-181127162316/85/Crystalization-Mass-Transfer-44-320.jpg)
![• Similarly,
• Some crystals little smaller than L2 would
become oversize in time dt, and leave the
range [L1, L2]
• Number of crystals in suspension volume V is
given below
• Number of crystals that crosses size L1 and
enters the range [L1, L2] over a time dt,](https://image.slidesharecdn.com/crystalization-181127162316/85/Crystalization-Mass-Transfer-45-320.jpg)
![• Number of crystals that crosses size L2 and
leaves the range [L1, L2] over a time dt,
• Feed source (inlet): crystal in feed in range
[L1,L2]
– Crystal in the range [L1,L2] may enter along with
the feed
• In exit stream:
– crystal in range [L1,L2] may leave the device with
suspension withdrawn as product.](https://image.slidesharecdn.com/crystalization-181127162316/85/Crystalization-Mass-Transfer-46-320.jpg)
![• Let ni –population density function of crystal
in feed.
• Average value of the density function in the
size range [L1,L2] is given by ñi
• If Qi – flow rate of liquor into crystallizer,
• Number of crystals entering with the feed in
time dt,](https://image.slidesharecdn.com/crystalization-181127162316/85/Crystalization-Mass-Transfer-47-320.jpg)
![• Let Qo - flow rate of product (exit) stream
with crystal size range [L1,L2] -
• Number of crystal in product stream is
• Writing a population balance equation for
crystal size range [L1,L2]](https://image.slidesharecdn.com/crystalization-181127162316/85/Crystalization-Mass-Transfer-48-320.jpg)
![• {No. of particles that grow and enter the size
range [L1,L2]
+ No. of particles that enter the vessel with
feed} =
• {No. of particles that grow (>L2) and leave the
range [L1,L2]
• + No. of particles that leave the vessel (with
product)}](https://image.slidesharecdn.com/crystalization-181127162316/85/Crystalization-Mass-Transfer-49-320.jpg)