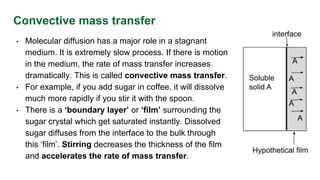
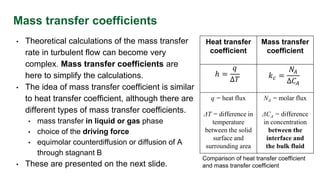
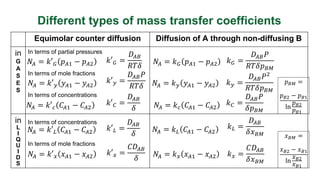
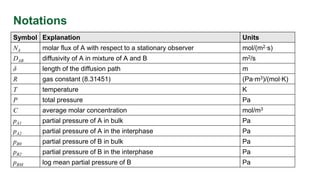
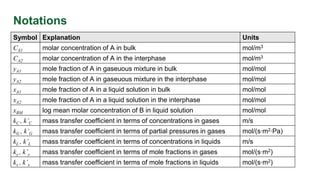
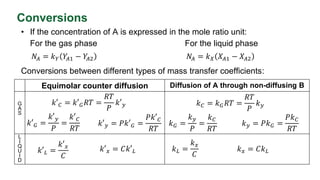
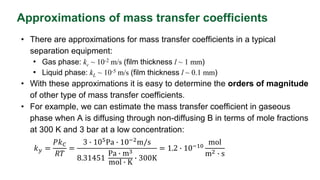
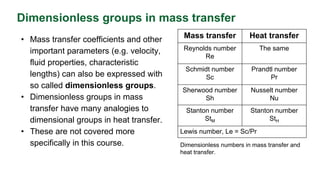
This document discusses convective mass transfer and mass transfer coefficients. It defines convective mass transfer as the rapid transfer of mass that occurs when there is motion in the transfer medium compared to the slower process of molecular diffusion. Mass transfer coefficients are introduced to simplify calculations of mass transfer rates. Different types of mass transfer coefficients are presented based on whether they are used for gases or liquids, and whether they are expressed in terms of concentrations, mole fractions, or partial pressures. Approximations for typical values of mass transfer coefficients in gas and liquid phases are provided.