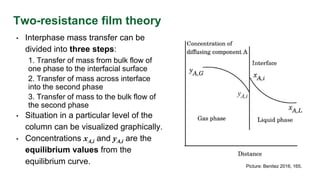
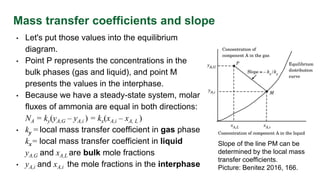
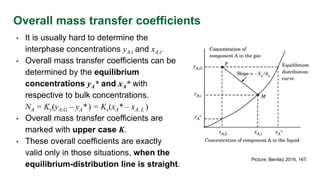
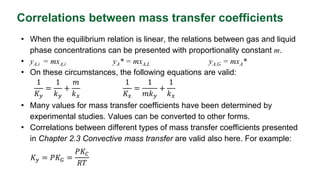
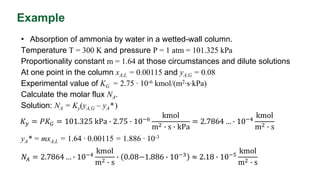
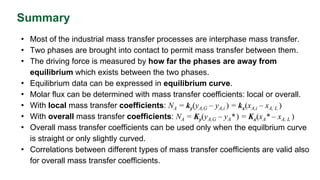
This document discusses interphase mass transfer, focusing on the processes of molecular and convective mass transfer between two phases. It emphasizes the importance of achieving equilibrium between these phases, the calculations of mass transfer coefficients, and how they can be used to determine molar flux in industrial applications. The overall mass transfer coefficients are applicable under specific conditions, and relationships between different types of coefficients are presented.