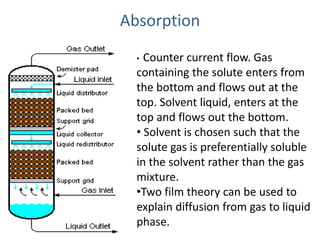
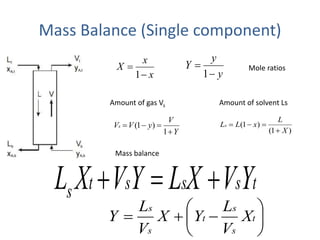
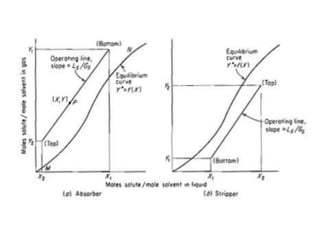
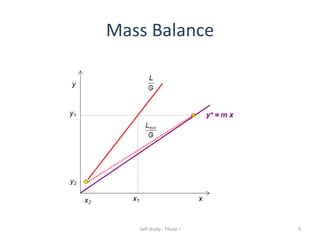
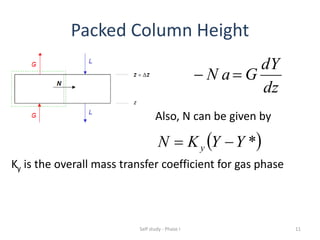
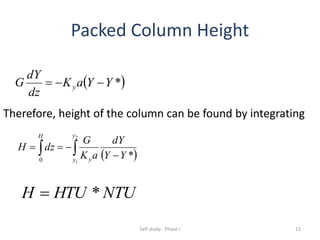
The document discusses mass transfer processes, specifically absorption and stripping in packed columns. It outlines the differences between packed and plate columns, solvent selection criteria, and mass balance equations for gas-liquid interactions. Additionally, it highlights applications in gas purification and removal of contaminants from various mediums.