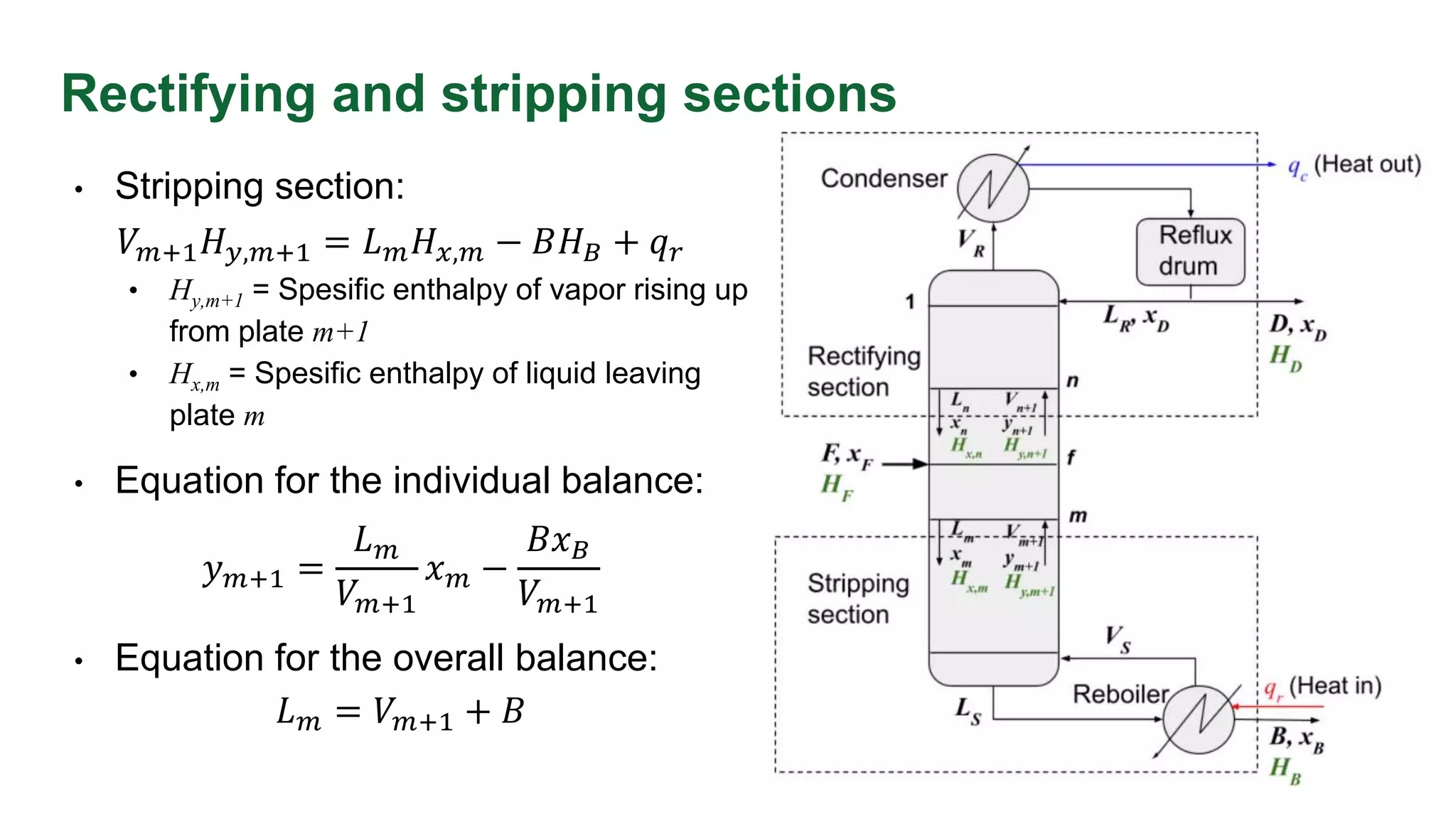
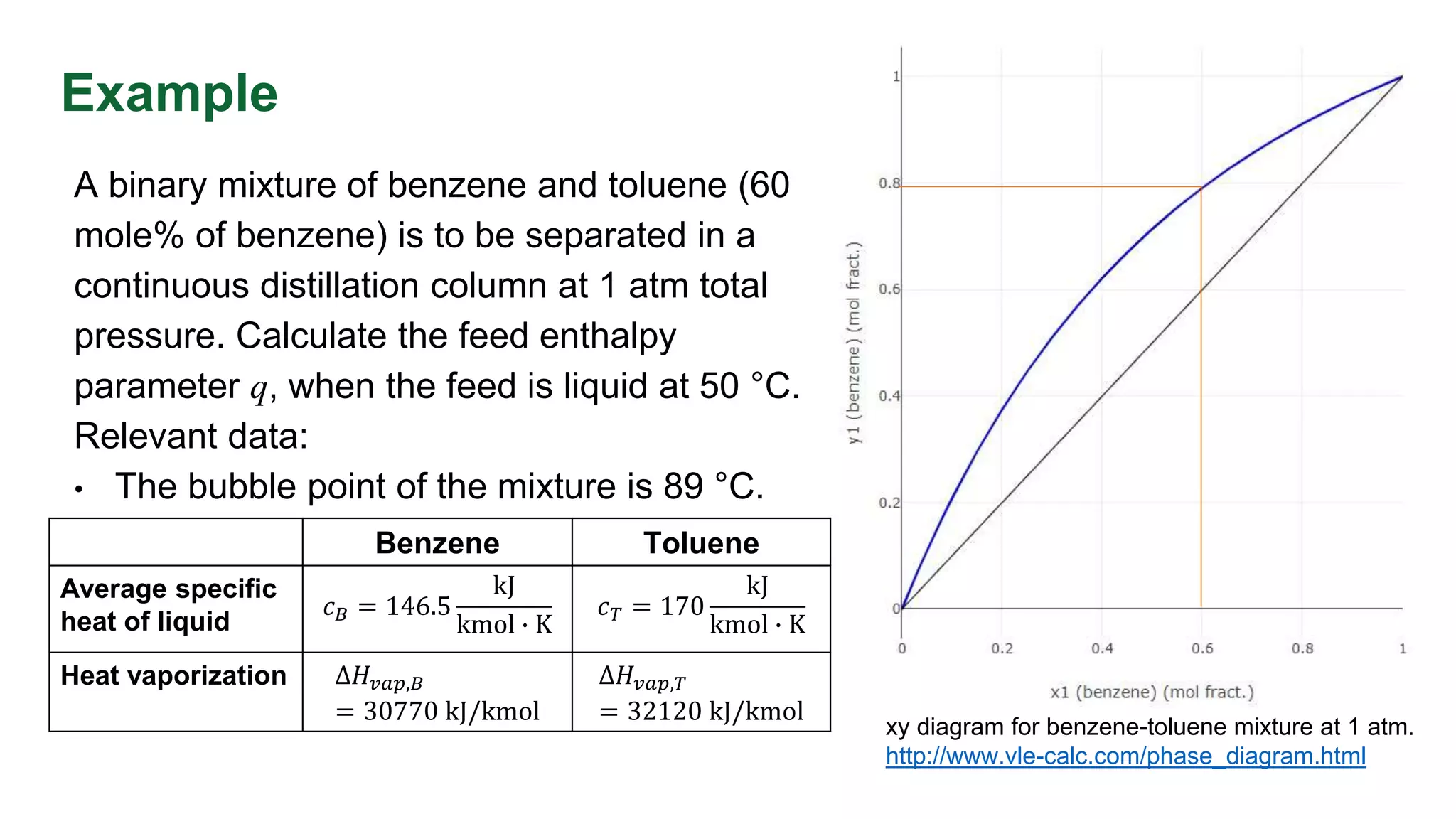
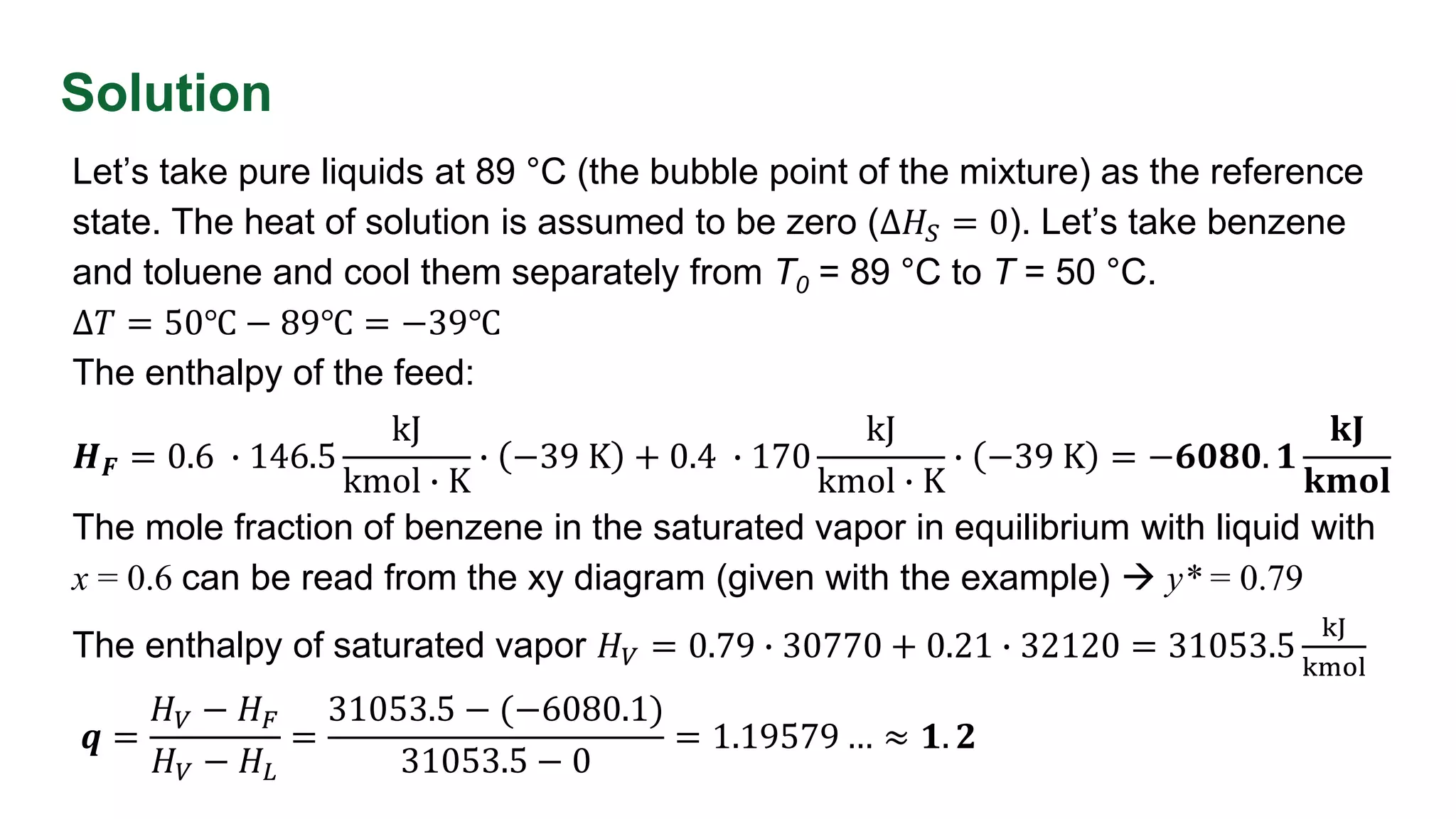
This document discusses enthalpy balances in distillation. It defines enthalpy and provides enthalpy-concentration diagrams. Equations for total and individual enthalpy balances are presented for the rectifying and stripping sections. The document describes how to solve the equations using an iterative approach with enthalpy data. It also discusses how to calculate the feed enthalpy parameter q using enthalpy values. An example problem calculates q for a benzene-toluene mixture.