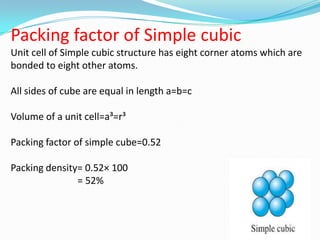
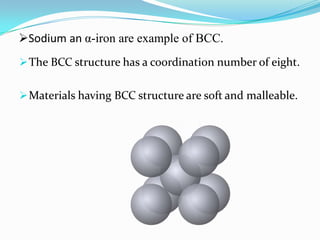
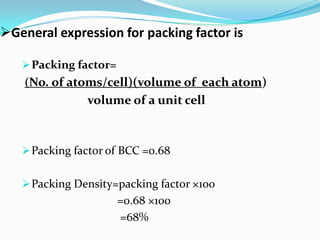
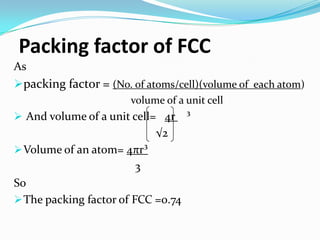
This document discusses packing factors for different crystal structures. It defines packing factor as the fraction of space occupied by atoms in a unit cell, assuming hard spheres. Simple cubic structure has a packing factor of 0.52 or 52%. Body centered cubic (BCC) has two atoms per unit cell, a packing factor of 0.68 or 68%. Face centered cubic (FCC) has four atoms per unit cell and the highest packing factor of 0.74 or 74%. Materials properties depend on their crystal structure and packing efficiency.