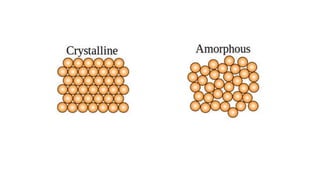
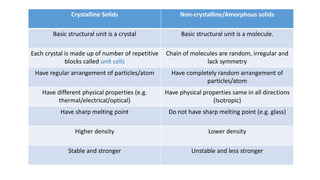
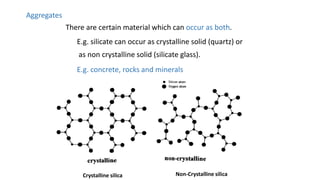
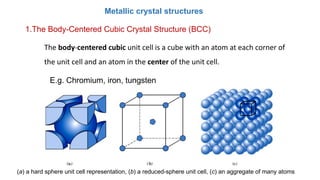
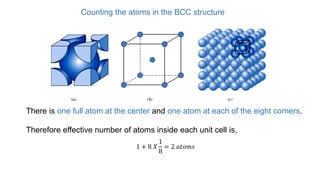
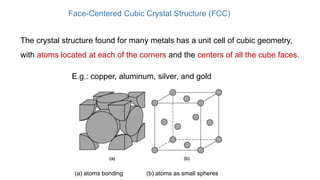
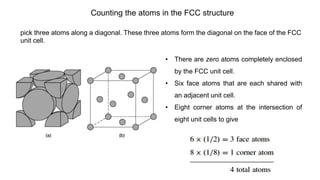
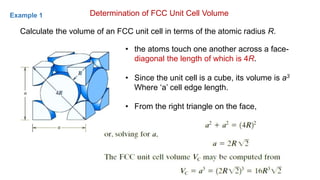
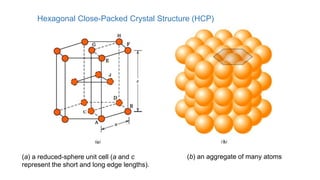
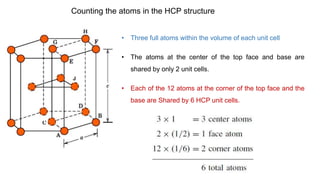
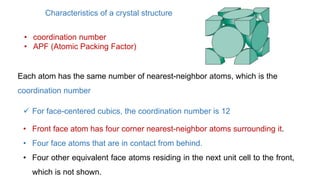
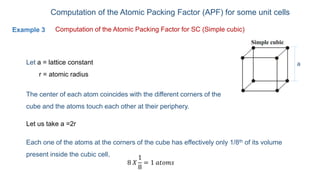
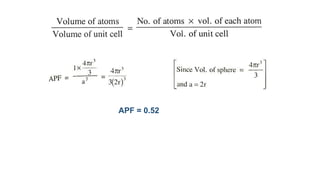
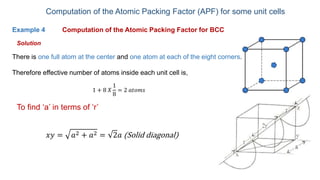
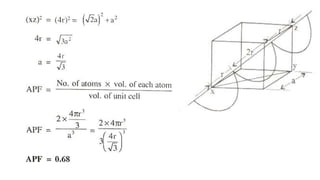
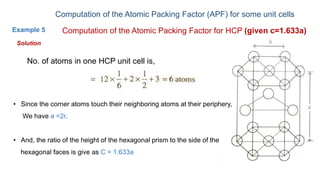
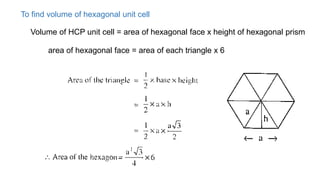
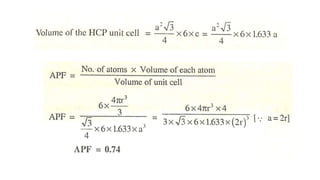
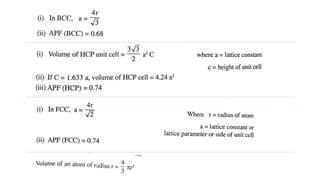
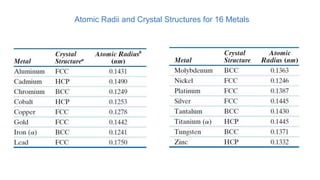
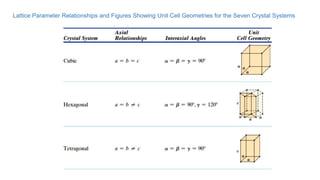
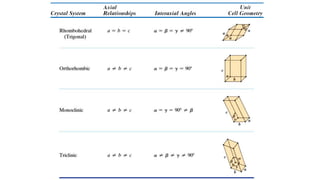
The document introduces the fundamentals of materials science and engineering, focusing on crystal structures such as body-centered cubic (bcc), face-centered cubic (fcc), and hexagonal close-packed (hcp) configurations. It explains the characteristics of crystalline vs. non-crystalline materials, emphasizing differences in atomic arrangement and physical properties. Additionally, it covers atomic packing factors, diffusion laws, and provides examples of various crystalline materials.