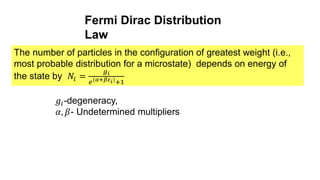
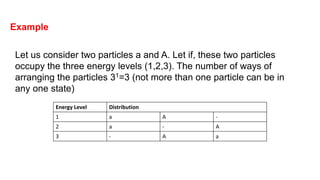
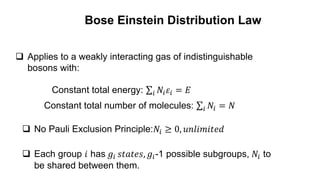
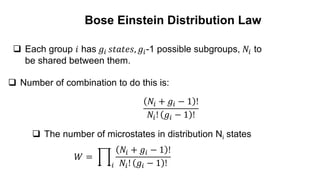
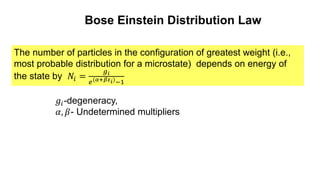
The document discusses three types of statistics that describe different physical situations: Maxwell-Boltzmann, Bose-Einstein, and Fermi-Dirac statistics. It outlines the characteristics, equations, and examples of each type, including the roles of distinguishability and energy occupancy rules for particles. Additionally, it touches on concepts such as Fermi energy and the derivation of the Fermi-Dirac and Bose-Einstein distribution laws.




![Maxwell Boltzmann Statistics (classical law)
This law states that, the total fixed amount of energy is distributed
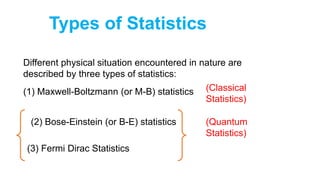
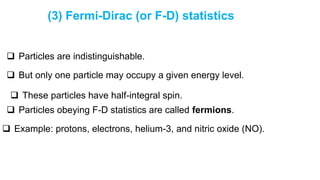
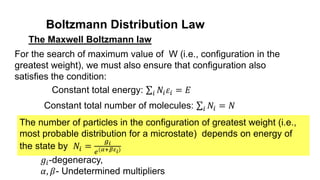
among various members of an assembly of identical particles.
𝜀0
𝜀1
𝜀2 Instantaneous configuration
of the system [5,0,0]
A general configuration [N0,N1,N2..] can be achieved in W
different ways, where W is called weight of the configuration.
W=
𝑁!
𝑁0!𝑁1!𝑁2!….](https://image.slidesharecdn.com/classicalquantumstatistics-230519024354-298d4c07/85/Classical-Quantum-Statistics-7-320.jpg)
![Question
Calculate the weight of the configuration in which 20 objects are
distributed in the arrangement 0, 1,5, 0, 8,0,3,2,0,1
Configuration =[0,1,5,0,8,0,3,2,0,1]
N =0+1+5+0+8+0+3+2+0+1= 20
Answer: 4.19 × 1010
W=
𝑁!
𝑁0!𝑁1!𝑁2!….](https://image.slidesharecdn.com/classicalquantumstatistics-230519024354-298d4c07/85/Classical-Quantum-Statistics-8-320.jpg)
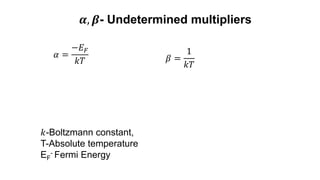
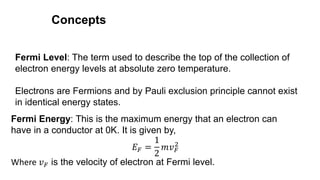



![Fermi Dirac Distribution-Derivation
Using Stirling’s approximation, one can eliminate the
factorial signs, yielding:
ln 𝑊 = 𝑖 ln 𝑊𝑖 = 𝑖 [𝑔𝑖ln 𝑔𝑖 − 𝑔𝑖 1 − 𝑓𝑖 𝑙𝑛(𝑔𝑖−𝑔𝑖𝑓𝑖) − 𝑔𝑖𝑓𝑖𝑙𝑛𝑔𝑖𝑓𝑖]
The total number of particles =N
The total energy of these N electrons = E
These system parameters are related to the number of states at
each energy, 𝑔𝑖 and the probability of occupancy of each state, 𝑓𝑖,
by:
N= 𝑖 𝑔𝑖𝑓𝑖 E= 𝑖 𝜀𝑖𝑔𝑖𝑓𝑖](https://image.slidesharecdn.com/classicalquantumstatistics-230519024354-298d4c07/85/Classical-Quantum-Statistics-15-320.jpg)