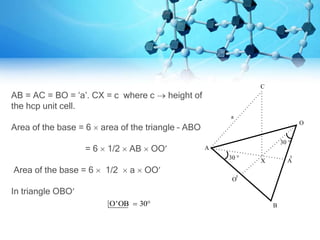
This document provides information on crystallography and the structure of crystalline solids. It defines key terms like crystalline solids, amorphous solids, space lattice, unit cell, and Bravais lattices. It describes the primary crystalline structures of metals including simple cubic, body centered cubic, face centered cubic, and hexagonal close packed. It provides details on the characteristics of each structure like atoms per unit cell, coordination number, and packing factor. Crystalline solids are described as having a regular orderly arrangement of atoms compared to the random arrangement in amorphous solids.




















![Simple Cubic
• A simple cubic unit structure consists of eight corner
atoms. It is a primitive cell.
• Lattice parameters:
a = b = c and α = β = γ = 900
• Effective number of atoms in unit cell:
• In actual crystals each and every corner atom is
shared by eight adjacent unit cells. There each and
every corner atom contributes 1/8 of its part to one
unit cell. Hence effective number of atoms in unit cell
= [1/8] X 8 = 1](https://image.slidesharecdn.com/crystallography-170204070427/85/Crystallography-23-320.jpg)

![• Atomic packing factor:
• A corner atom is shared by eight unit cells
• Contribution of a corner atom is 1/8
• Cube has 8 corners
• Hence contribution of 8 corner atoms= [1/8]X8 = 1
• Number of atoms per unit cell= 1
• If r is the radius of the atom, distance between the
centers of two neighboring atoms = 2r = a
Atomic radius r = a/2
• Volume of one atom = 4/3 πr3
• Volume of unit cell = a3](https://image.slidesharecdn.com/crystallography-170204070427/85/Crystallography-25-320.jpg)

![Body Centered Cubic
Body centered cubic structure consists of eight corner atoms and
one body centered atom. It is not a primitive cell.
Lattice parameters: a = b = c and α = β = γ = 900
Effective number of atoms in unit cell:
In BCC unit cell, each and every corner atom is shared by
eight adjacent unit cells. Total number of atoms contributed by
corner atoms = [1/8] X 8 = 1
BCC unit cell has 1 full atom at the center of the unit cell.
The effective number of atoms present in a bcc unit cell is =1+1 = 2](https://image.slidesharecdn.com/crystallography-170204070427/85/Crystallography-27-320.jpg)