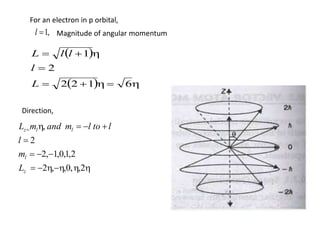
The vector atom model quantizes both the magnitude and direction of electron orbits in atoms. It introduces a reference axis, such as the direction of an external magnetic field, to specify the orientation of electron orbits in space. According to this model, the direction of electron orbits is restricted to a few preferred directions determined by any external forces, like a magnetic field. Both the magnitude and direction of orbits are quantized, making the orbits vector quantities. The model also accounts for the electron spin, which is another quantized vector that contributes to the total angular momentum and magnetic moment of the atom.