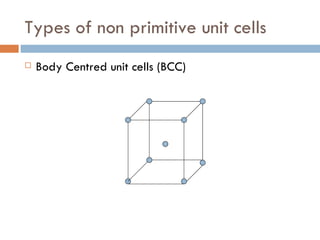
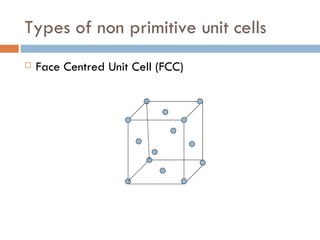
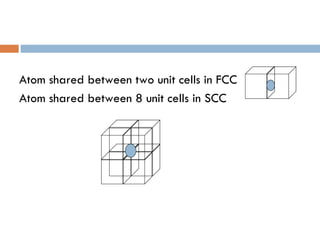
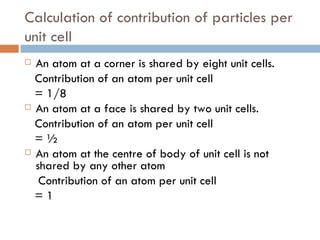
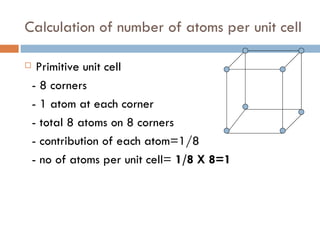
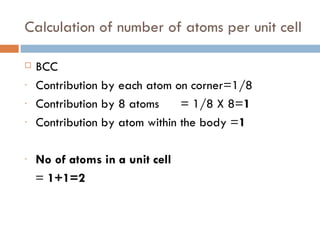
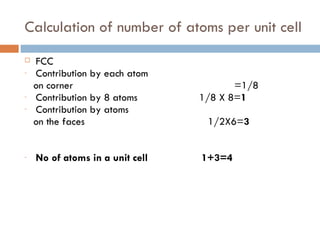
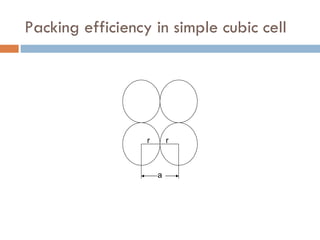
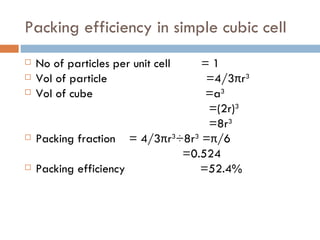
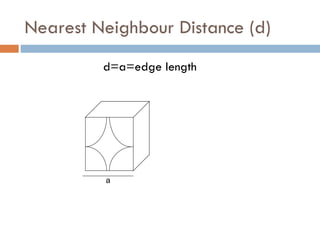
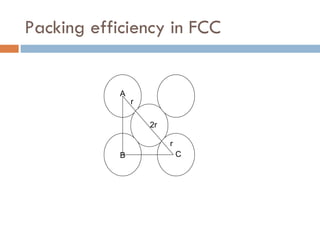
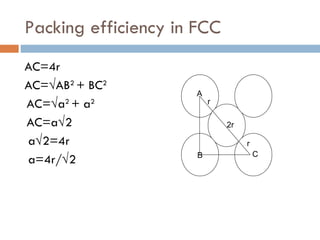
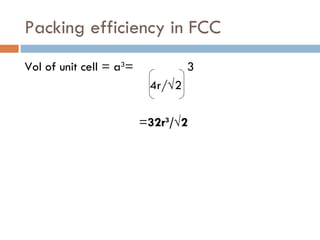
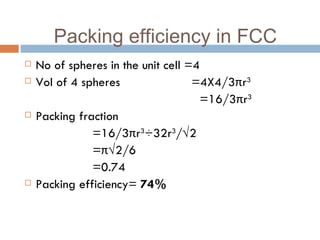
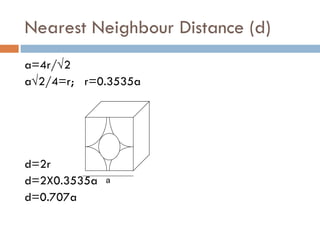
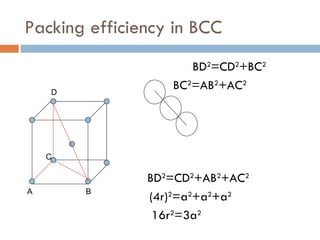
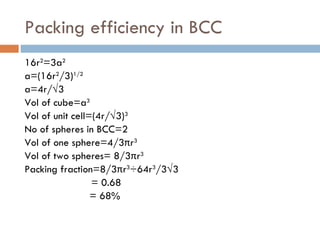
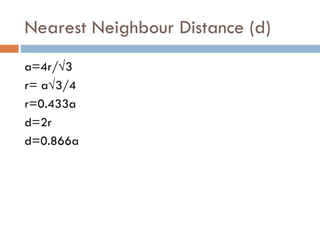
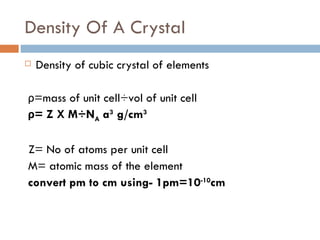
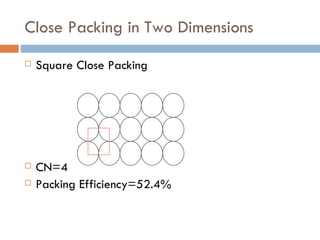
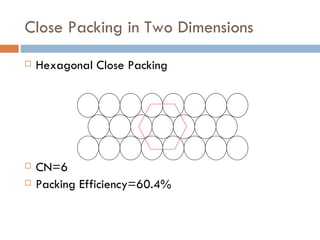
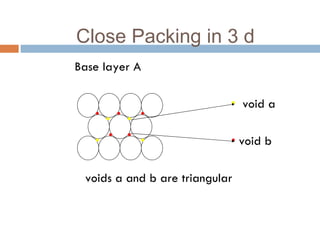
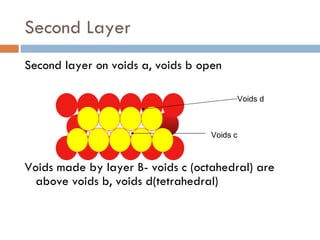
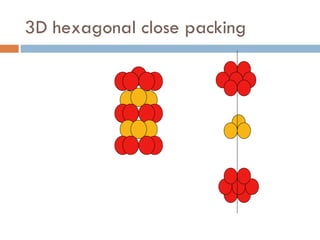
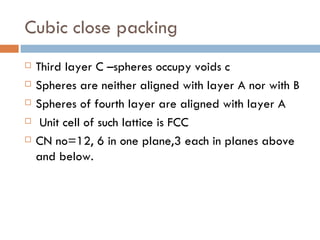
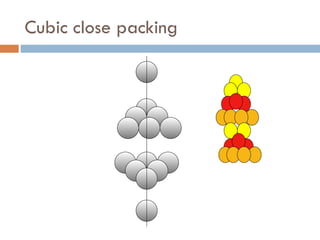
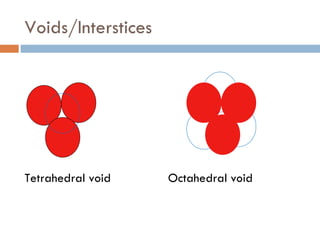
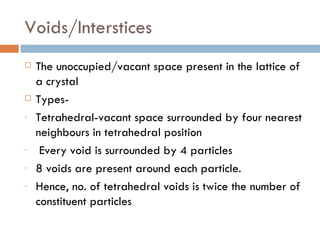
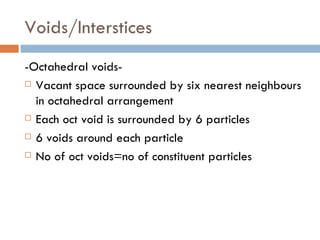
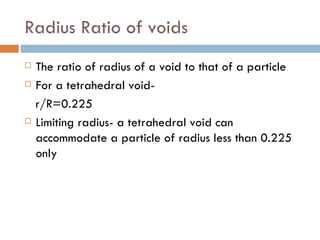
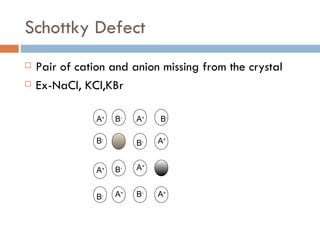
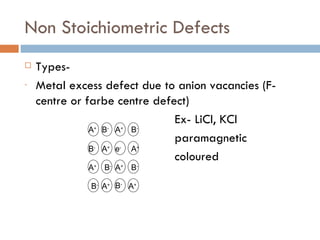
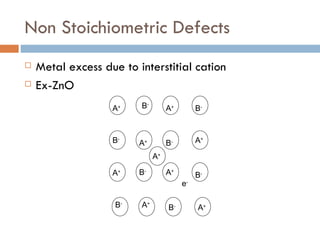
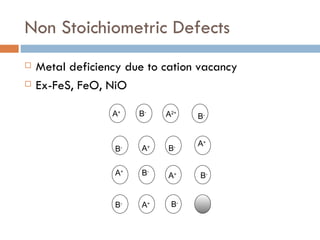
The document discusses different types of crystal defects including point defects, stoichiometric defects, and non-stoichiometric defects. Stoichiometric defects include Schottky and Frenkel defects which involve cation-anion pairs missing or cation dislocations. Non-stoichiometric defects result from deviations from the ideal ratio of cations to anions and include metal excess or deficiency defects involving anion or cation vacancies. Common examples of different defect types in various crystals are provided.