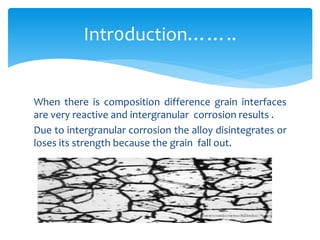
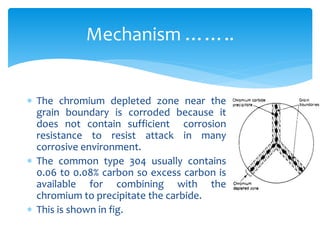
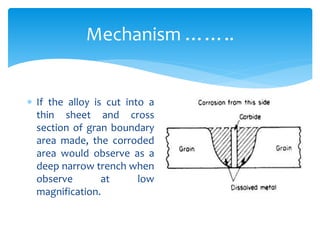
Intergranular corrosion is a localized corrosion that occurs at grain boundaries of metals, leading to the disintegration of alloys and loss of strength. It can be caused by impurities, variations in alloy composition, and precipitation of intermetallic compounds, especially in environments like welding. Prevention involves using low carbon content steel and proper heat treatment to minimize carbide formation.