The document summarizes the 8 main forms of corrosion that can occur in metals. It begins by explaining that corrosion is a natural process that converts refined metals back into more stable forms, driven by thermodynamics. It then describes the key elements that form an electrochemical corrosion cell and discusses various factors that influence corrosion rates. The main types of corrosion covered are uniform corrosion, localized corrosion (including galvanic and crevice corrosion), and stress corrosion cracking. Visual examples of each type of corrosion are provided.



 [2Fe2O3 · xH2O](s) + 8H+
(aq)
The resulting iron oxide, Fe2O3, is one of the most stable states for iron to be found in.
This simple example of an electrochemical reaction demonstrates one of the most
important principles of metallic corrosion: the rate of oxidation equals the rate of
reduction.3
It is important to note that when alloys are corroded more than one oxidation
and one reduction reaction can occur.
Another way of looking at the corrosion process is as a series of anodic and
cathodic reactions. In this situation four elements are necessary for corrosion to occur:
• An anode.
• A cathode.
• An electrolyte (water)
• A metallic path for electron flow.
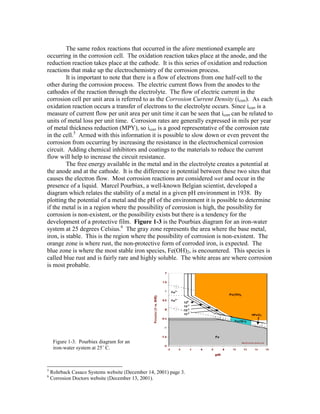
When all four of these elements are present a corrosion cell is formed. It is
important to remember that the anodic and cathodic reactions do not have to occur at the
same spot on the material. As long as an electrolyte and a metallic path exist, these half-
cells can exist and corrosion will occur. Figure 1-2 is an example of the corrosion cell
with the four elements labeled.4
2
Davis, Neil T. (2001) page 37.
3
Fontana, Mars G. (1986) page 15.
4
Allen, Thomas O., Roberts, Alan P. (1993) page 10-1, 10-2.
Figure 1-2. Corrosion
cell diagram depicting
the current flow from the
corrosion pit.](https://image.slidesharecdn.com/eightformsofcorrosion-160326114731/85/Eight-forms-of-corrosion-4-320.jpg)