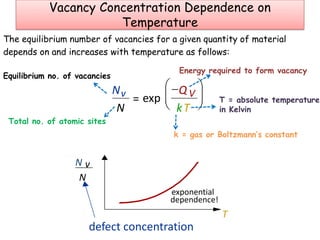
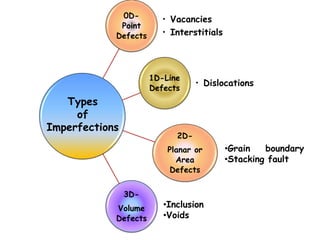
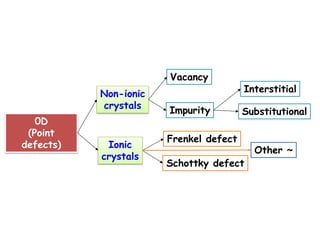
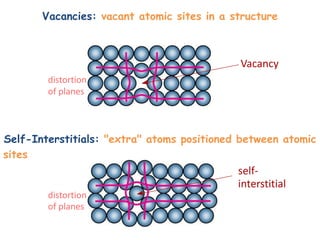
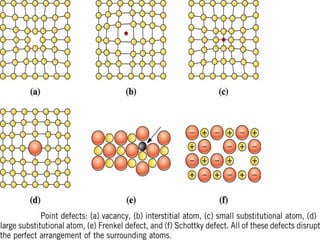
Point defects in solids include vacancies, interstitials, and impurities. Vacancies are vacant atomic sites, while interstitials are atoms that occupy spaces between normal atomic sites. Common point defects include vacancies, self-interstitials, Schottky defects, and Frenkel defects. The concentration of intrinsic point defects like vacancies increases exponentially with temperature based on the energy required to form the defect. Point defects can also create color centers where defects cause colors like the green color from vacancies in diamond.





![Thermodynamics of intrinsic defects
Formation of a vacancy- missing bonds and distortion of the lattice
Potential energy (Enthalpy) of the system increases
Work required for the formation of a point defect →
Enthalpy of formation (Hf) [kJ/mol or eV/defect]
n defects are distributed over N lattice sites
W possible arrangements
Now and
Therefore,
For minimum
For n << N
0
n
G
n
nN
kT
H f
ln
kT
H
N
n f
exp](https://image.slidesharecdn.com/m2-pointdefects-140820002737-phpapp02/85/M2-point-defects-13-320.jpg)