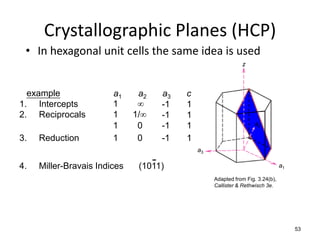
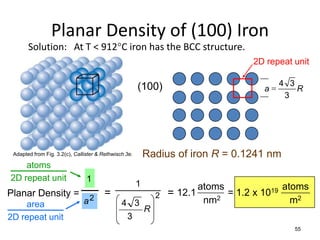
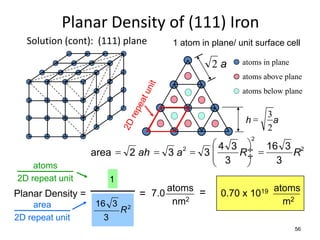
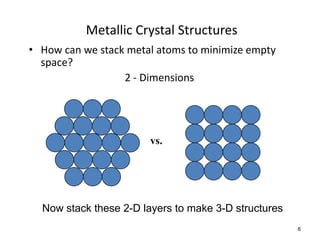
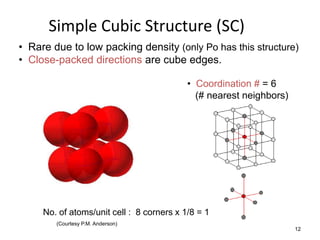
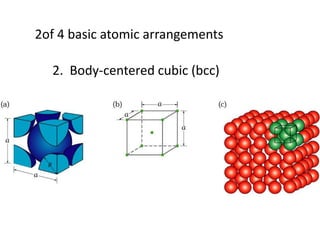
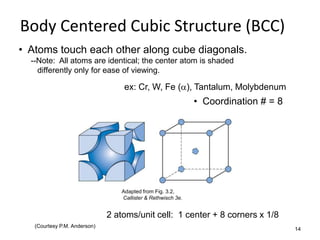
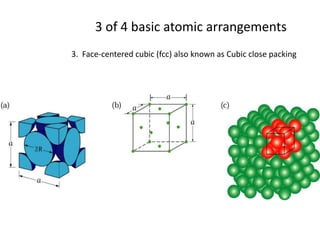
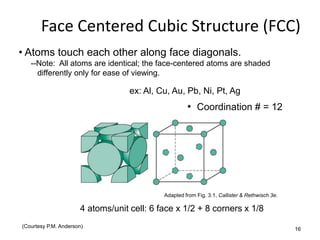
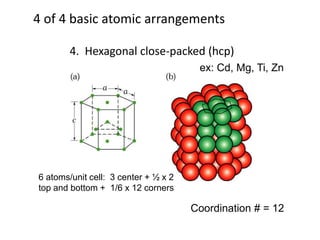
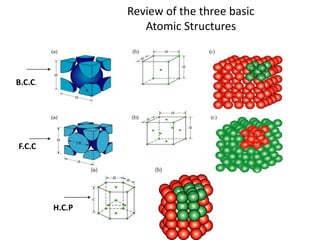
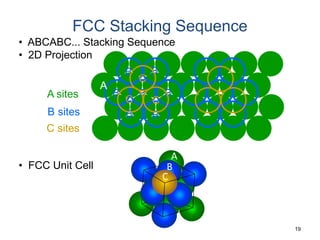
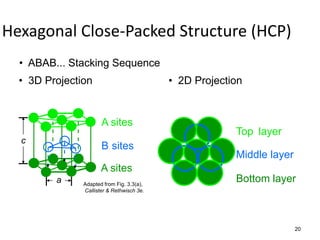
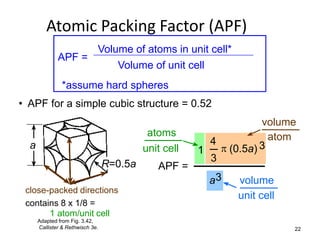
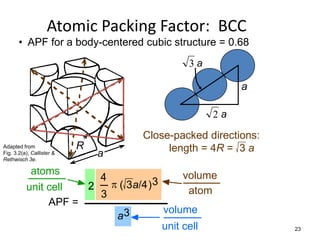
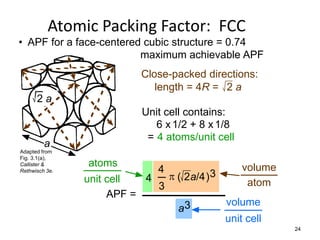
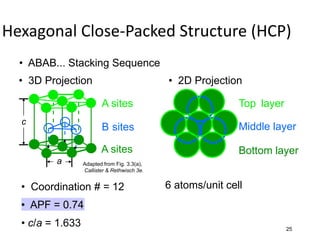
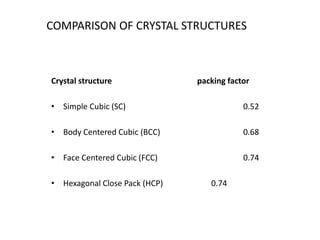
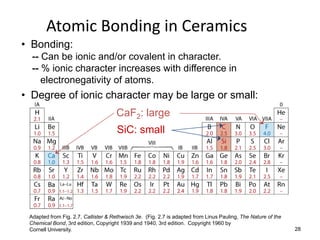
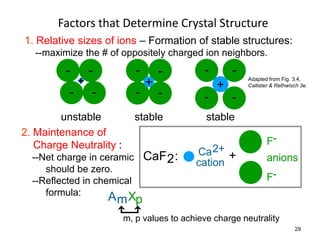
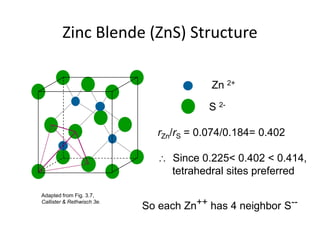
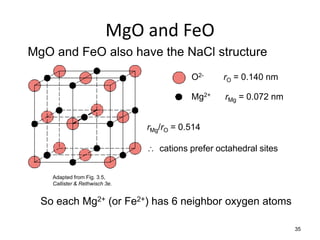
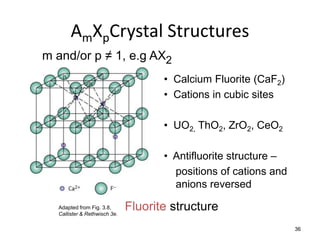
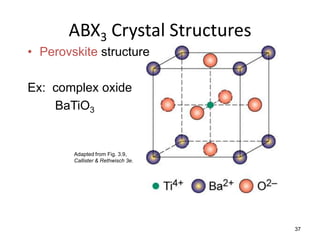
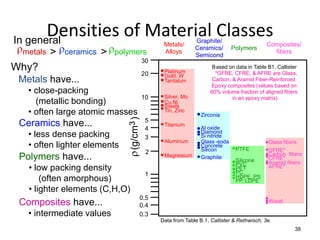
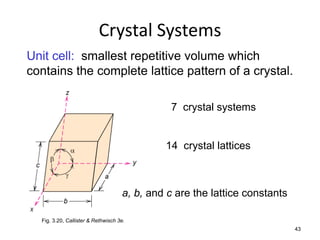
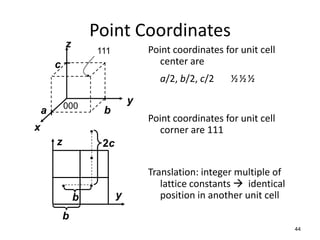
There are four basic atomic arrangements that determine the properties of metals: simple cubic, body-centered cubic, face-centered cubic, and hexagonal close-packed. The atomic packing factor, which represents the fraction of unit cell volume occupied by atoms, increases in the order of simple cubic, body-centered cubic, and face-centered cubic structures. Face-centered cubic has the highest atomic packing factor of 0.74 and is therefore the most dense arrangement. Different metallic crystal structures can explain variations in density and other material properties between metals.







![Single vs Polycrystals
• Single Crystals E (diagonal) = 273 GPa
Data from Table 3.7,
-Properties vary with Callister & Rethwisch
3e. (Source of data is
direction: anisotropic. R.W. Hertzberg,
Deformation and
-Example: the modulus Fracture Mechanics of
Engineering Materials,
of elasticity (E) in BCC iron: 3rd ed., John Wiley and
Sons, 1989.)
E (edge) = 125 GPa
• Polycrystals
-Properties may/may not 200 mm Adapted from Fig.
5.19(b), Callister &
vary with direction. Rethwisch 3e.
(Fig. 5.19(b) is courtesy
-If grains are randomly of L.C. Smith and C.
Brady, the National
oriented: isotropic. Bureau of Standards,
Washington, DC [now
(Epoly iron = 210 GPa) the National Institute of
Standards and
-If grains are textured, Technology,
anisotropic. Gaithersburg, MD].)
41](https://image.slidesharecdn.com/crystalsystems-130121012543-phpapp02/85/Crystal-systems-41-320.jpg)
![Crystallographic Directions
z Algorithm
1. Vector repositioned (if necessary) to pass
through origin.
2. Read off projections in terms of
unit cell dimensions a, b, and c
y 3. Adjust to smallest integer values
4. Enclose in square brackets, no commas
x [uvw]
ex: 1, 0, ½ => 2, 0, 1 => [ 201 ]
-1, 1, 1 => [ 111 ] where overbar represents a
negative index
families of directions <uvw>
45](https://image.slidesharecdn.com/crystalsystems-130121012543-phpapp02/85/Crystal-systems-45-320.jpg)
![Linear Density
Number of atoms
• Linear Density of Atoms LD =
Unit length of direction vector
[110]
ex: linear density of Al in [110]
direction
a = 0.405 nm
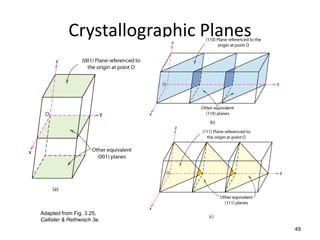
# atoms
a 2
LD 3.5 nm 1
length 2a
46](https://image.slidesharecdn.com/crystalsystems-130121012543-phpapp02/85/Crystal-systems-46-320.jpg)
![HCP Crystallographic Directions
z
Algorithm
1. Vector repositioned (if necessary) to pass
through origin.
2. Read off projections in terms of unit
a2 cell dimensions a1, a2, a3, or c
3. Adjust to smallest integer values
- 4. Enclose in square brackets, no commas
a3
[uvtw] a
a1
2
Adapted from Fig. 3.24(a),
Callister & Rethwisch 3e.
a2 -a3
2
ex: ½, ½, -1, 0 => [ 1120 ] a3
a1
2
dashed red lines indicate
projections onto a1 and a2 axes a1
47](https://image.slidesharecdn.com/crystalsystems-130121012543-phpapp02/85/Crystal-systems-47-320.jpg)
![HCP Crystallographic Directions
• Hexagonal Crystals
– 4 parameter Miller-Bravais lattice coordinates are
related to the direction indices (i.e., u'v'w') as follows.
z
[ u 'v 'w ' ] [ uvtw ]
1
u (2 u ' - v ')
3
a2
1
v (2 v ' - u ')
- 3
a3
t - (u +v )
w w'
a1
Fig. 3.24(a), Callister & Rethwisch 3e.
48](https://image.slidesharecdn.com/crystalsystems-130121012543-phpapp02/85/Crystal-systems-48-320.jpg)