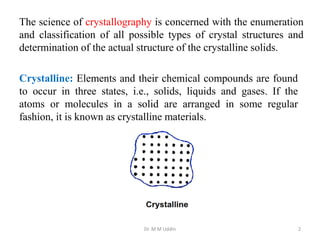
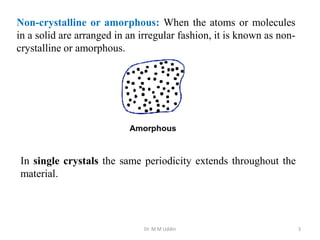
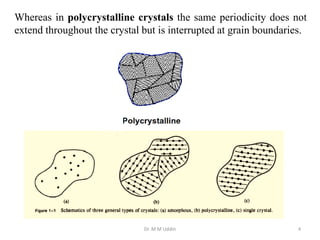
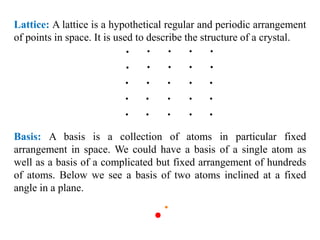
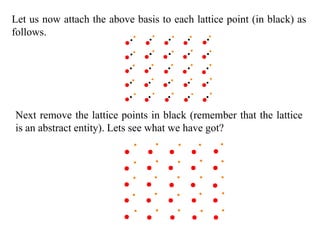
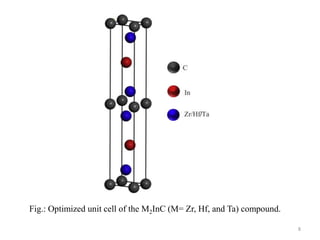
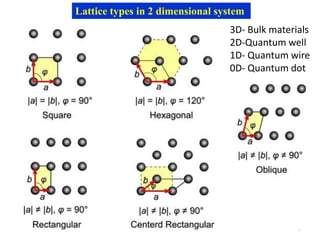
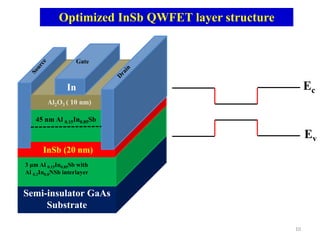
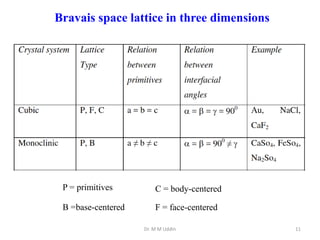
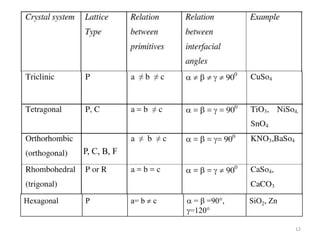
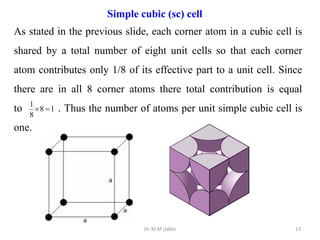
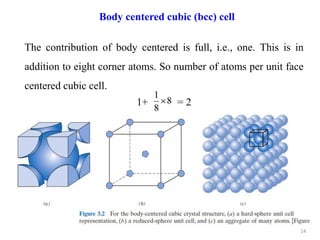
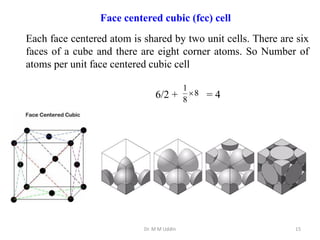
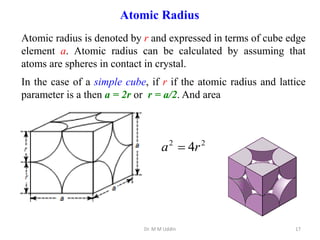
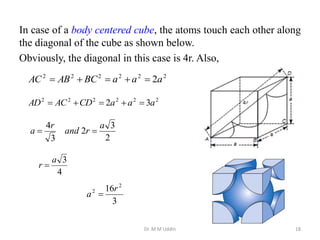
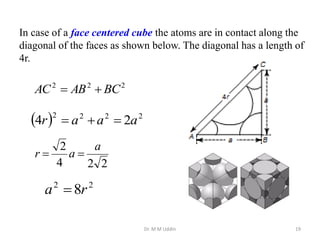
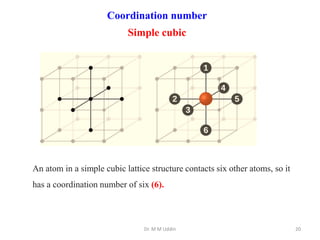
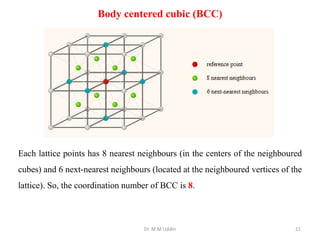
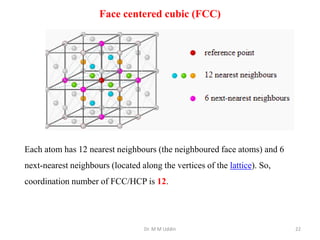
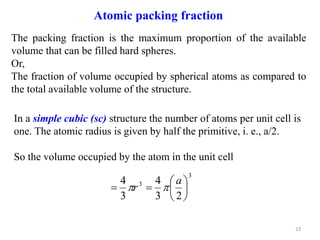
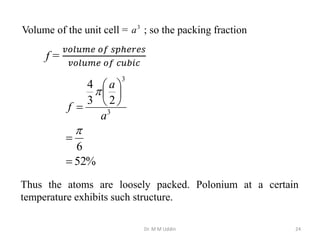
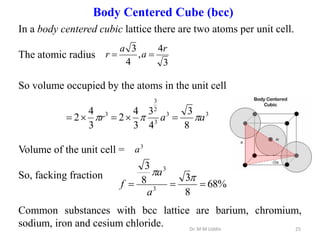
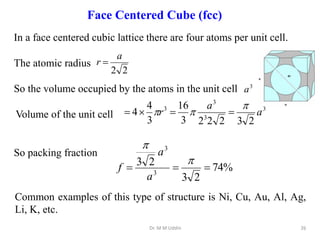
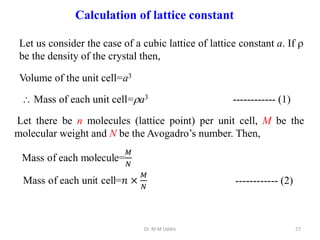
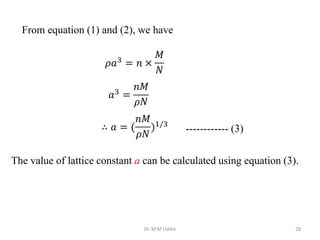
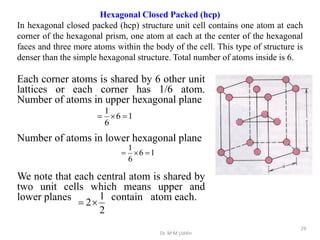
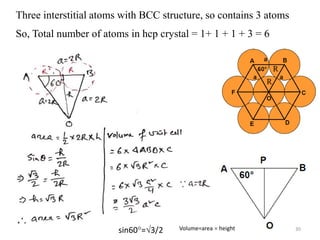
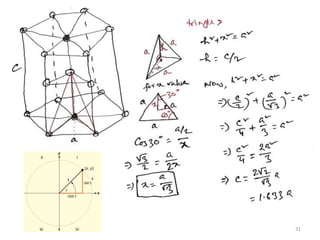
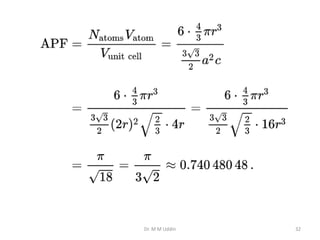
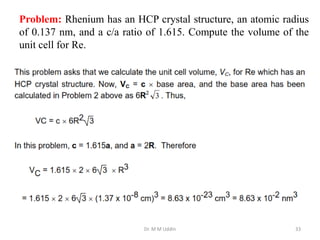
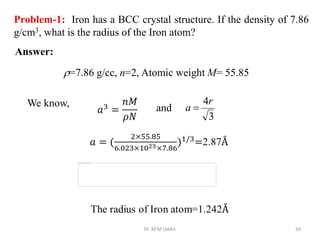
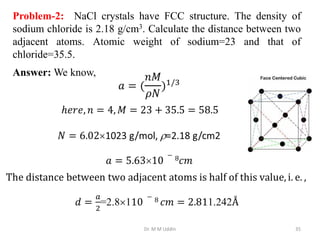
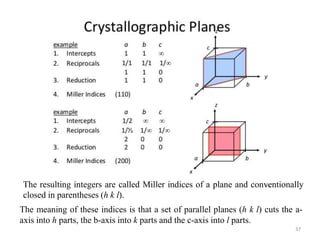
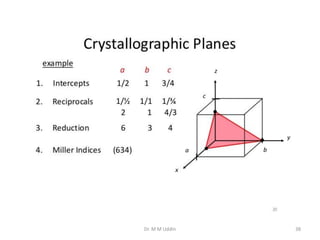
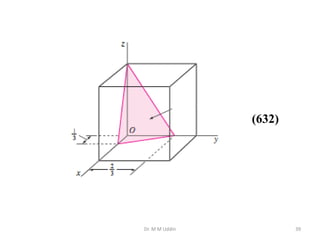
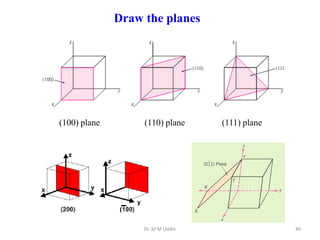
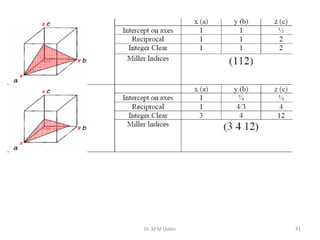
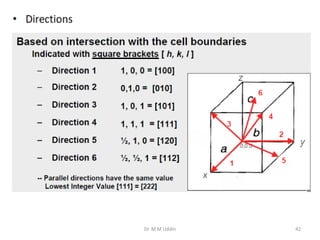
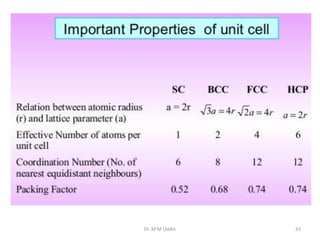
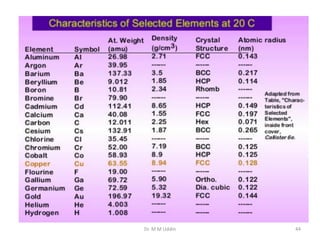
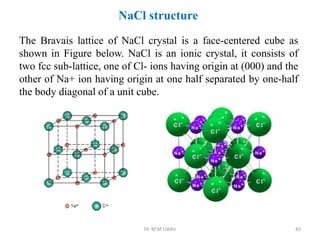
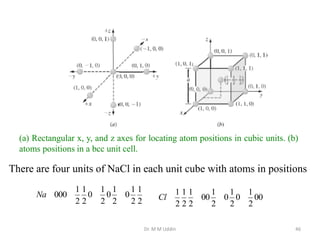
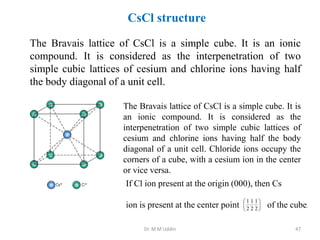
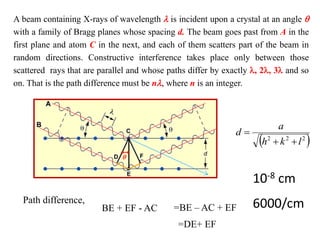
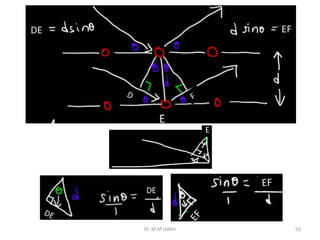
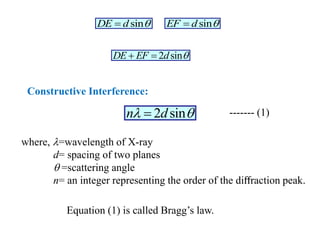
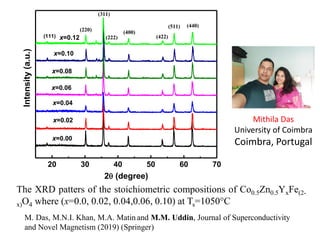
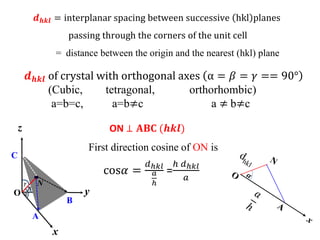
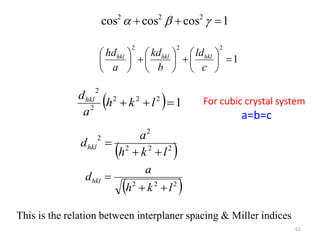
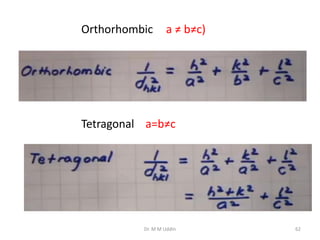
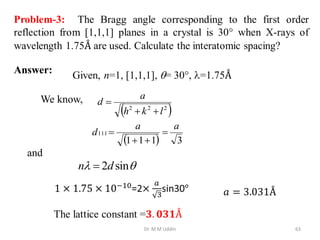
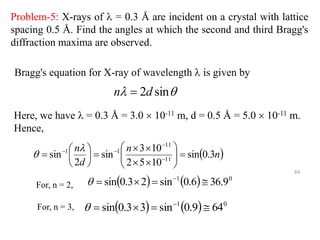
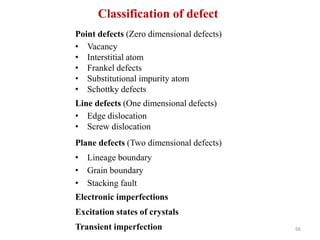
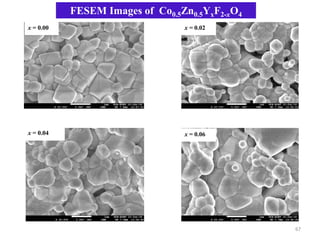
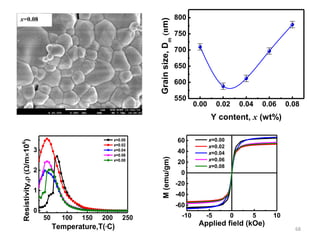
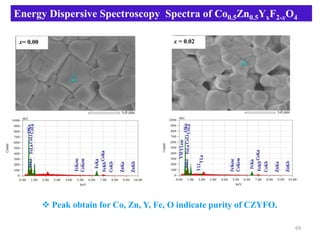
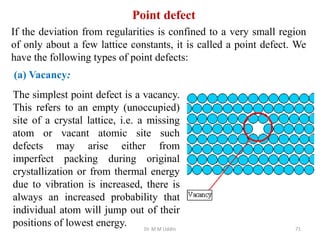
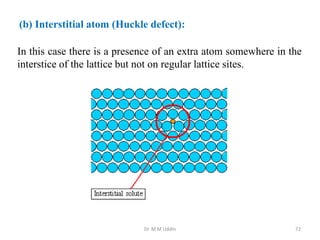
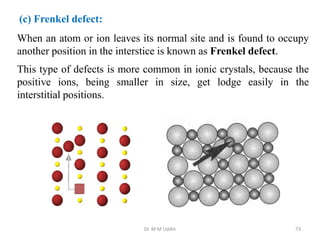
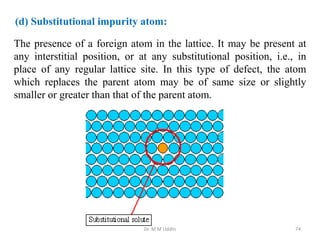
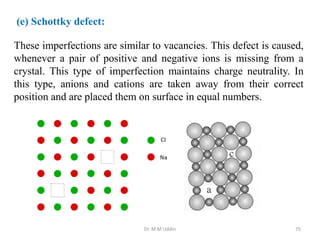
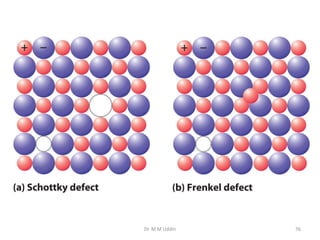
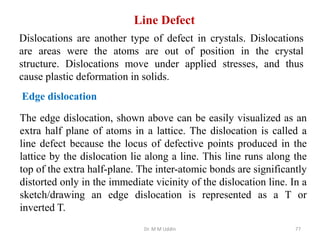
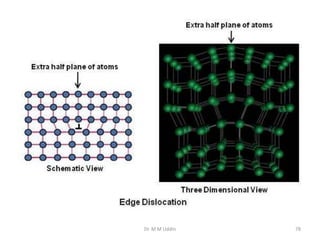
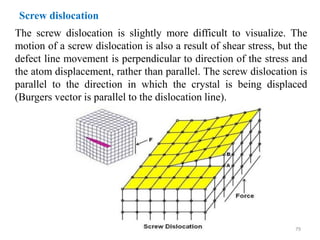
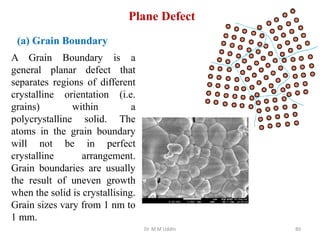
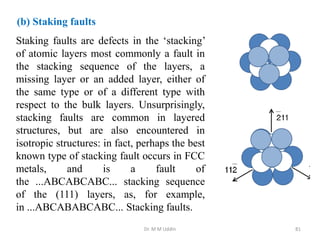
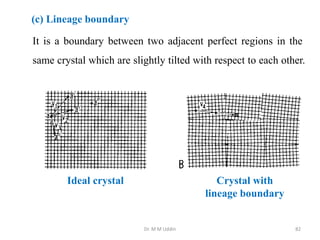
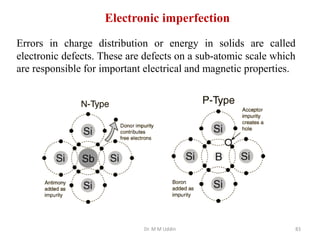
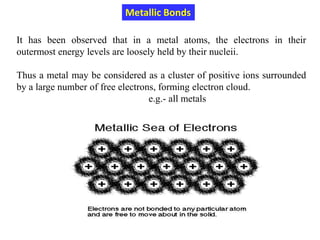
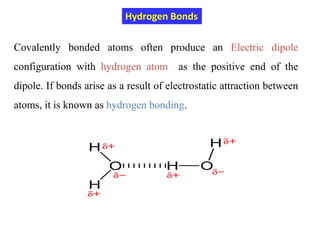
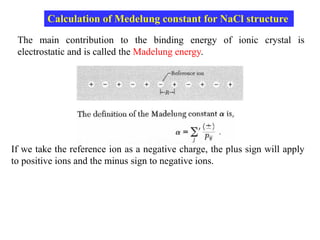
This document provides an introduction to solid state physics and crystallography. It defines key terms like crystalline, non-crystalline, lattice, basis, unit cell and discusses common crystal structures including simple cubic, body centered cubic, and face centered cubic. It also covers topics like Miller indices, atomic packing fraction, coordination number, and examples of crystal structures in sodium chloride and cesium chloride.