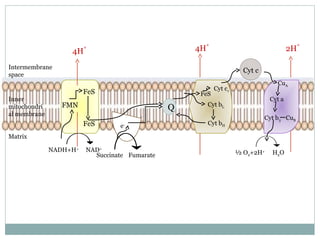
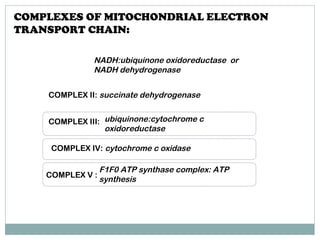
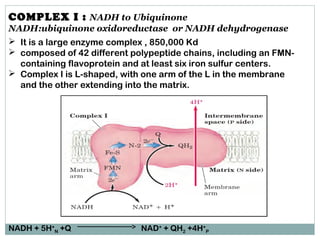
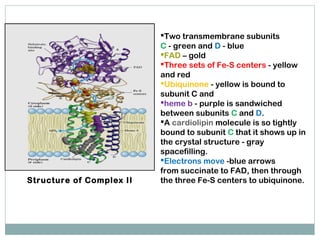
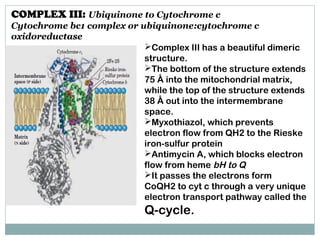
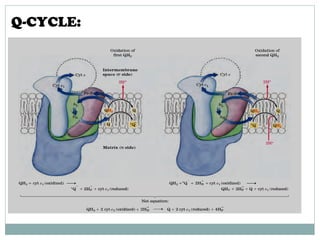
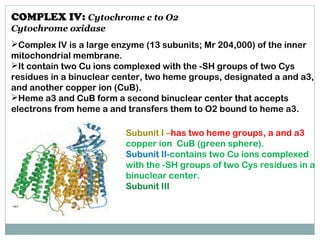
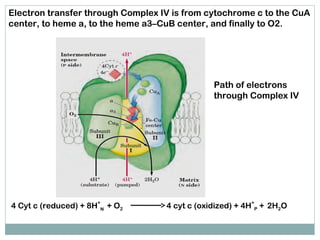
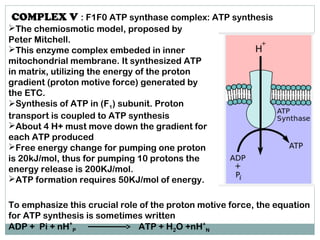
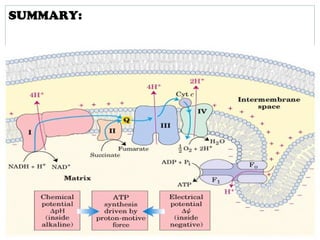
The document summarizes the organization of the mitochondrial electron transport chain. It describes the five complexes of the electron transport chain (Complexes I-V), including their components, functions, and electron transfer processes. Specifically, it details how Complexes I, III, and IV transfer electrons from donors like NADH to final acceptors like oxygen. This generates a proton gradient across the inner mitochondrial membrane, which Complex V then uses to synthesize ATP through oxidative phosphorylation.