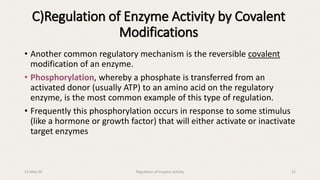
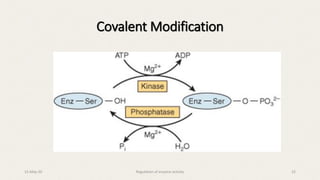
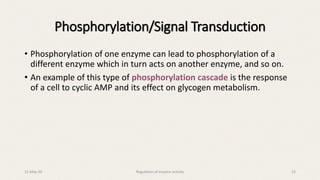
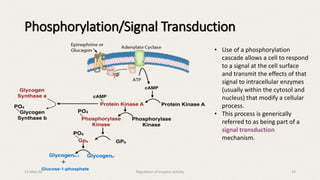
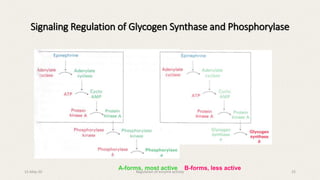
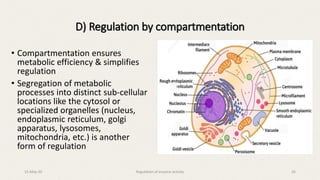
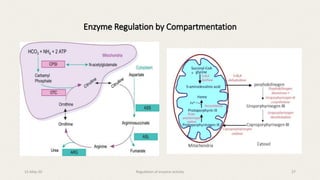
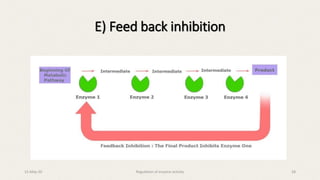
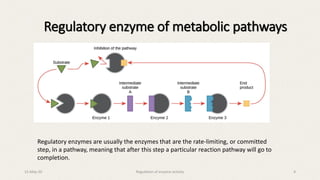
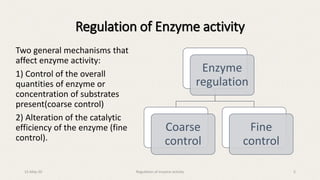
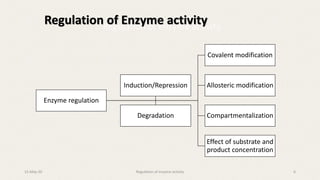
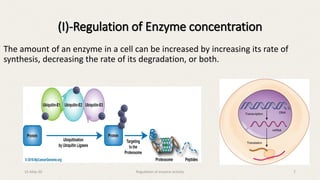
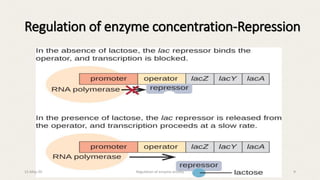
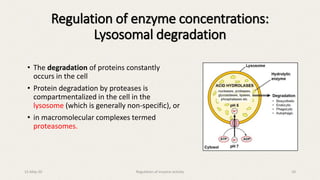
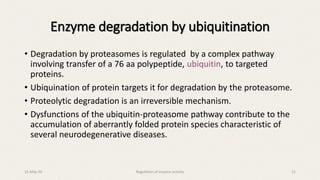
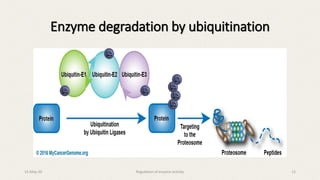
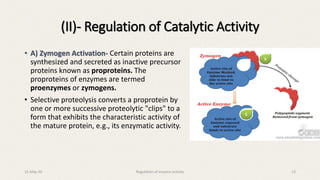
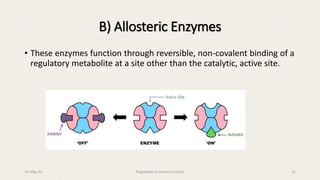
The document discusses the regulation of enzyme activity essential for maintaining homeostasis and how dysregulation can lead to diseases like cancer and diabetes. It outlines mechanisms of enzyme regulation, including coarse and fine control, zymogen activation, allosteric regulation, covalent modifications, and compartmentation. Additionally, it highlights the significance of feedback inhibition in metabolic processes.






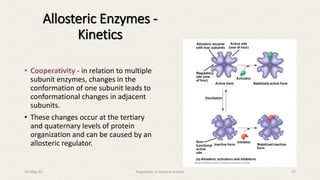
![• Allosteric enzymes do exhibit saturation kinetics
at high [S], but they have a characteristic
sigmoidal saturation curve rather than
hyperbolic curve when vo is plotted versus [S]
(analogous to the oxygen saturation curves of
myoglobin vs. hemoglobin).
• The sigmoidicity is thought to result from the
cooperativity of structural changes between
enzyme subunits (again similar to oxygen binding
to hemoglobin).
Allosteric Enzymes - Kinetics
15-May-20 Regulation of enzyme activity 19](https://image.slidesharecdn.com/enzymeregulation-200514235921/85/Enzyme-regulation-19-320.jpg)
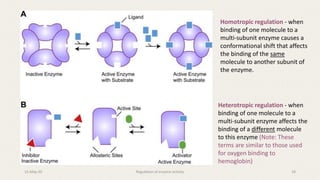
![Vo v/s [S] for Allosteric Enzymes
Addition of an allosteric activator (+)
tends to shift the curve to a more
hyperbolic profile (more like
Michaelis-Menten curves), while an
allosteric inhibitor (-) will result in
more pronounced sigmoidal curves.
15-May-20 Regulation of enzyme activity 20](https://image.slidesharecdn.com/enzymeregulation-200514235921/85/Enzyme-regulation-20-320.jpg)