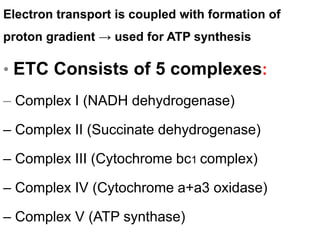
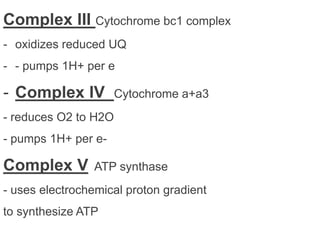
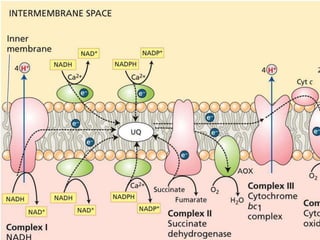
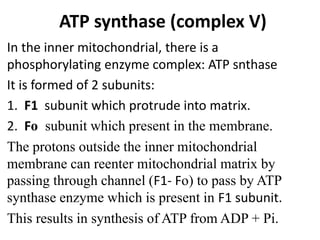
The electron transport chain is the final pathway where electrons from nutrients are transferred to oxygen to form water. Electrons enter the chain from NADH or FADH2 and are passed through four complexes and coenzyme Q, which pump protons out of the mitochondrial matrix. This creates a proton gradient that is used by ATP synthase to phosphorylate ADP and make ATP via oxidative phosphorylation. The chemiosmotic hypothesis explains this coupling of electron transport to ATP synthesis.