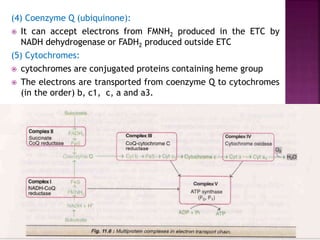
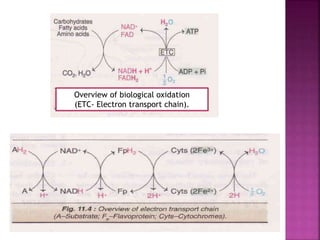
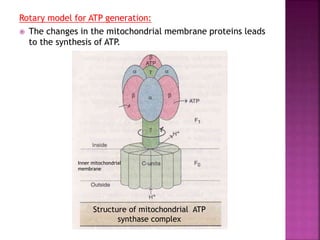
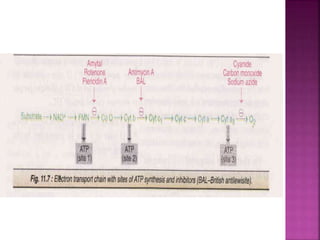
The document discusses the electron transport chain and oxidative phosphorylation. It describes how electrons from nutrients are transferred through enzyme complexes in the mitochondrial membrane to generate a proton gradient. This gradient is then used by ATP synthase to phosphorylate ADP into ATP through oxidative phosphorylation. Inhibitors of the electron transport chain like cyanide and antimycin A prevent this process, while uncouplers allow electron transport without phosphorylation.