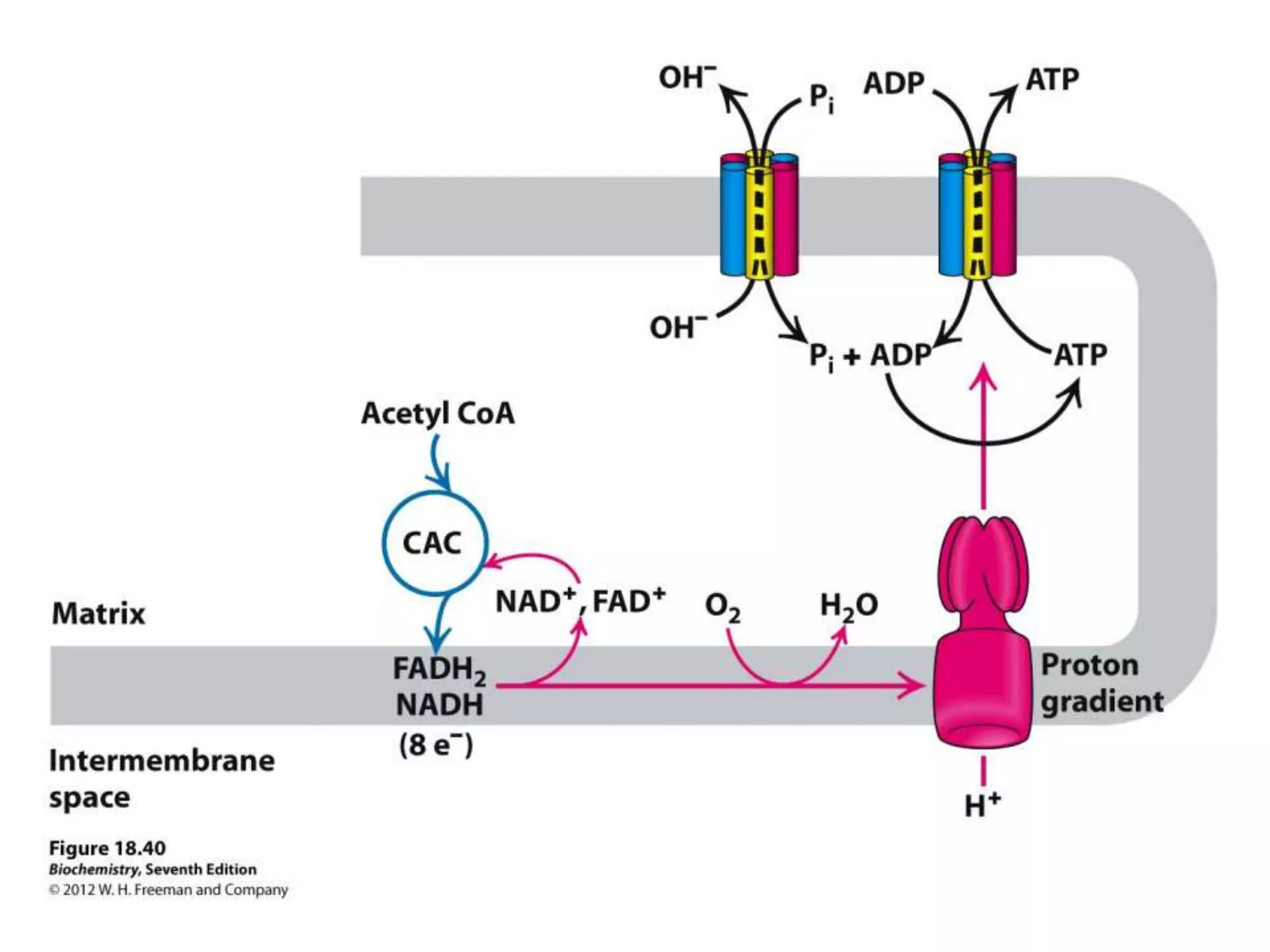
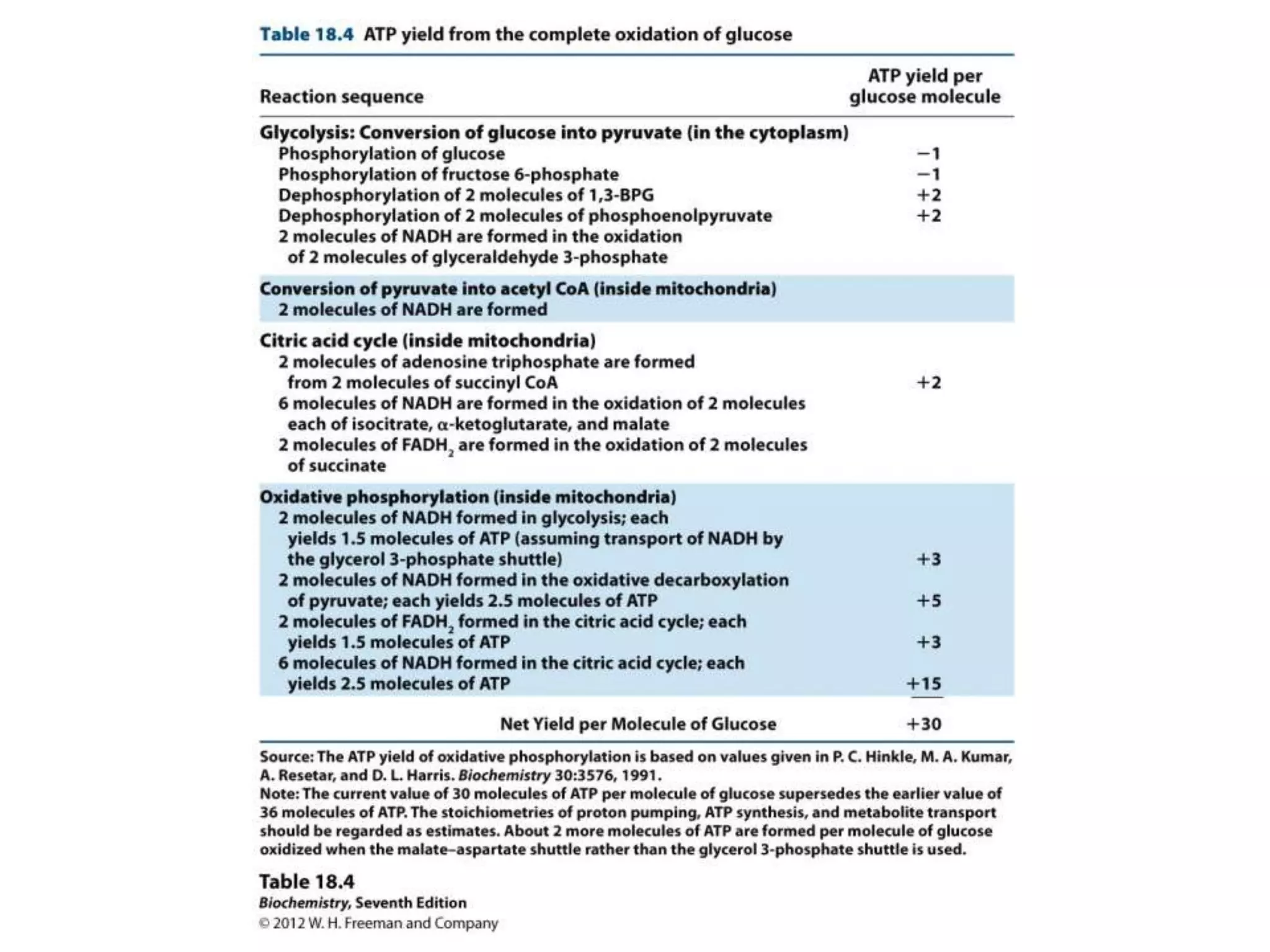
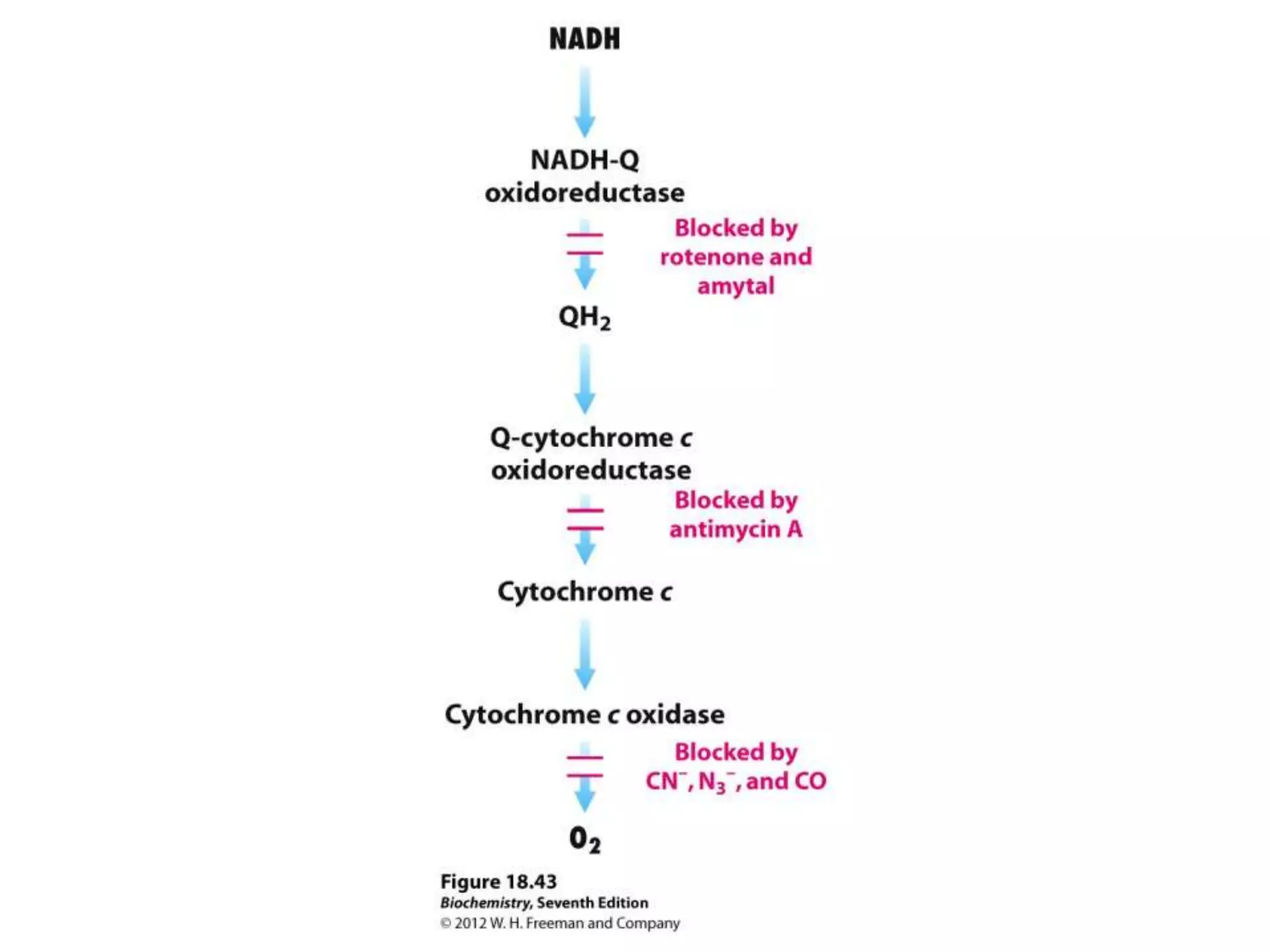
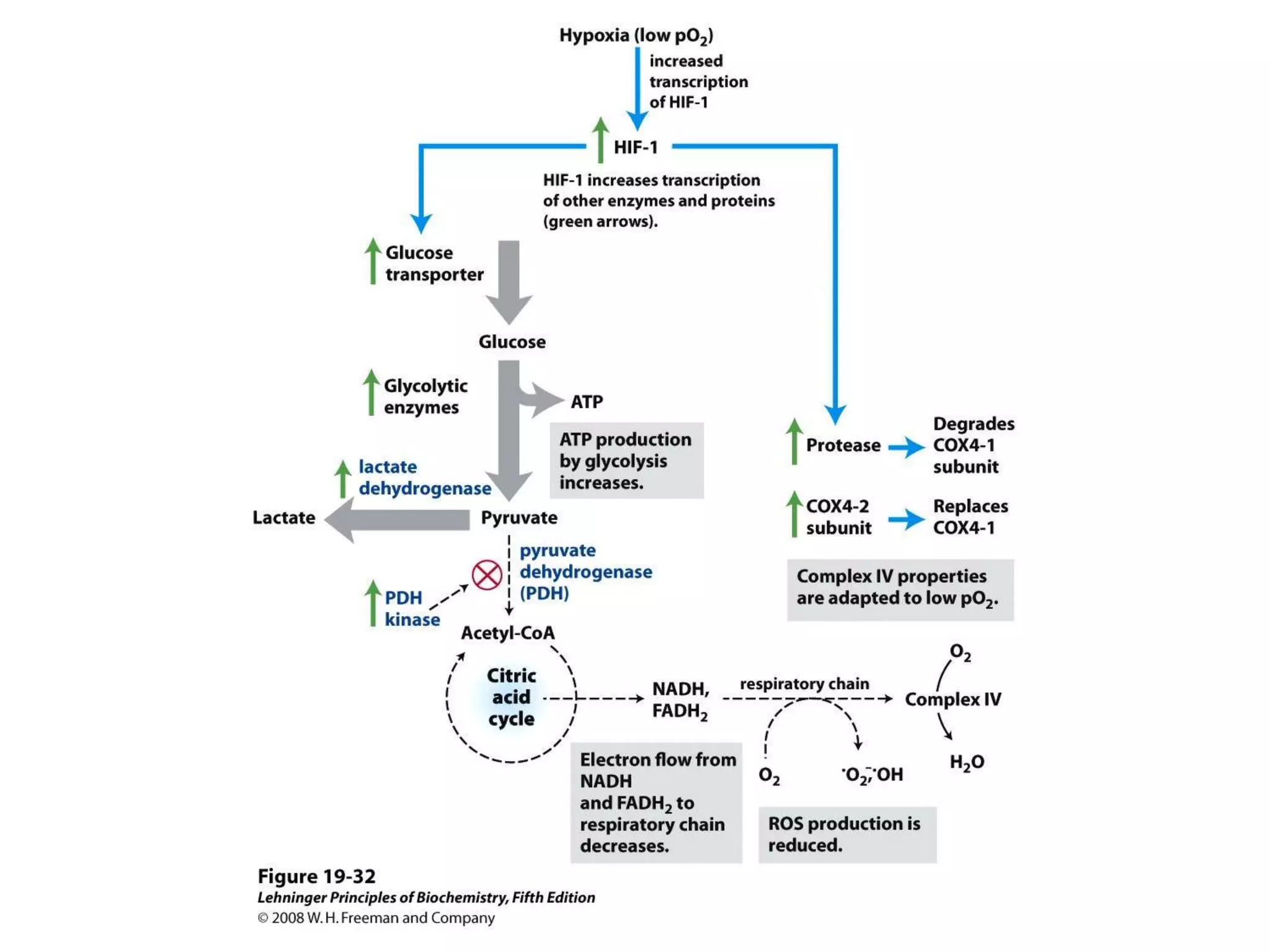
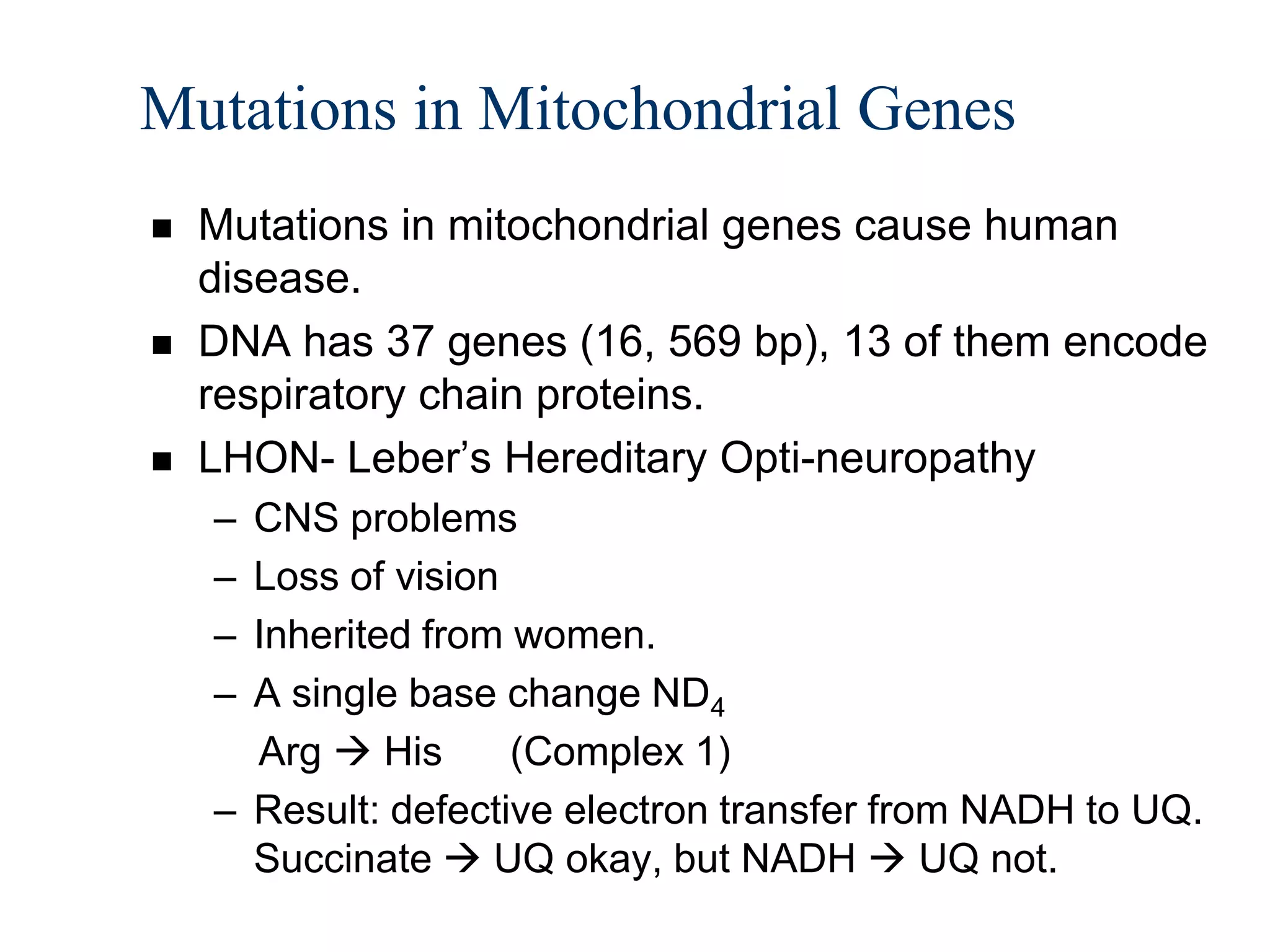
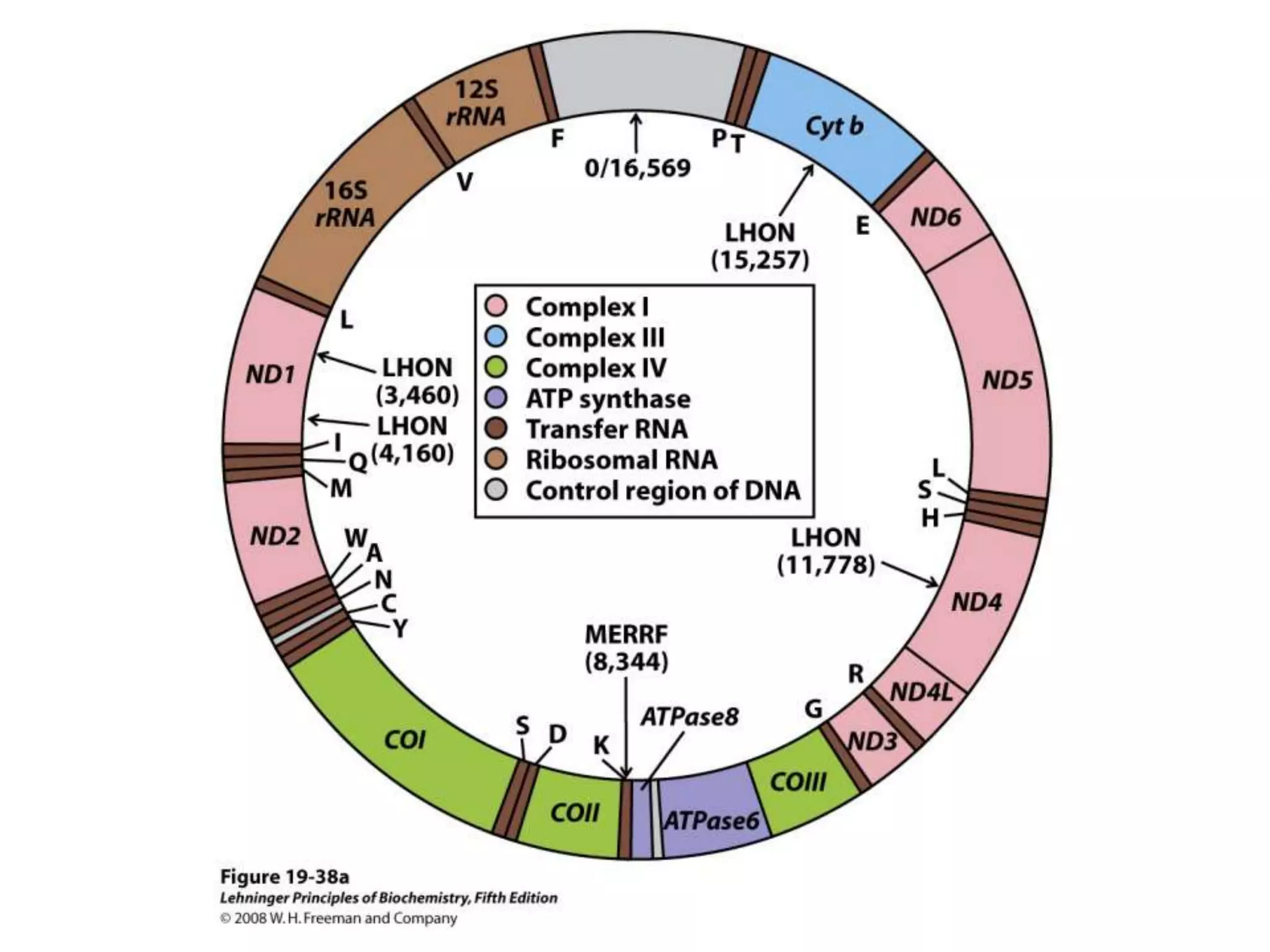
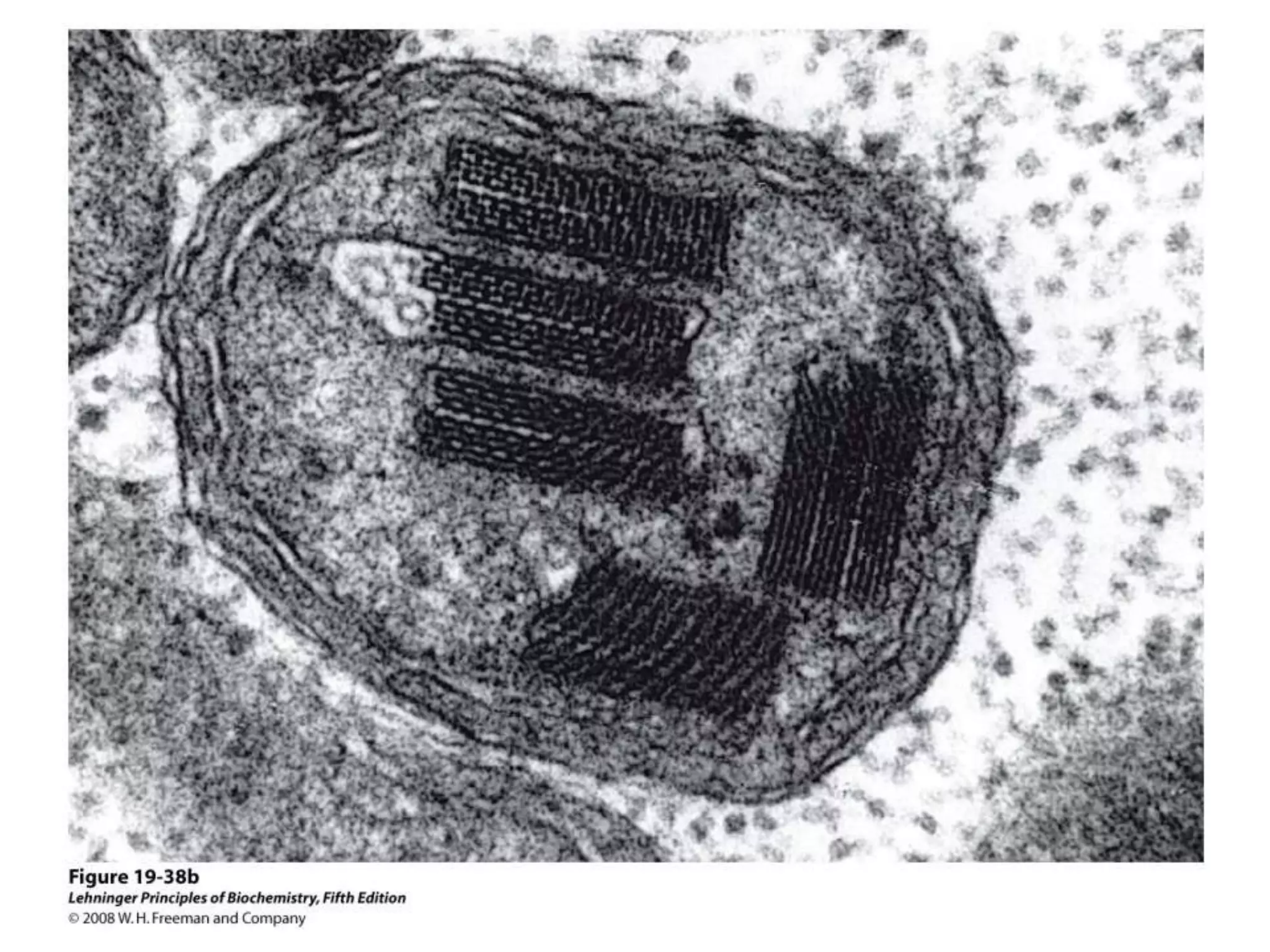
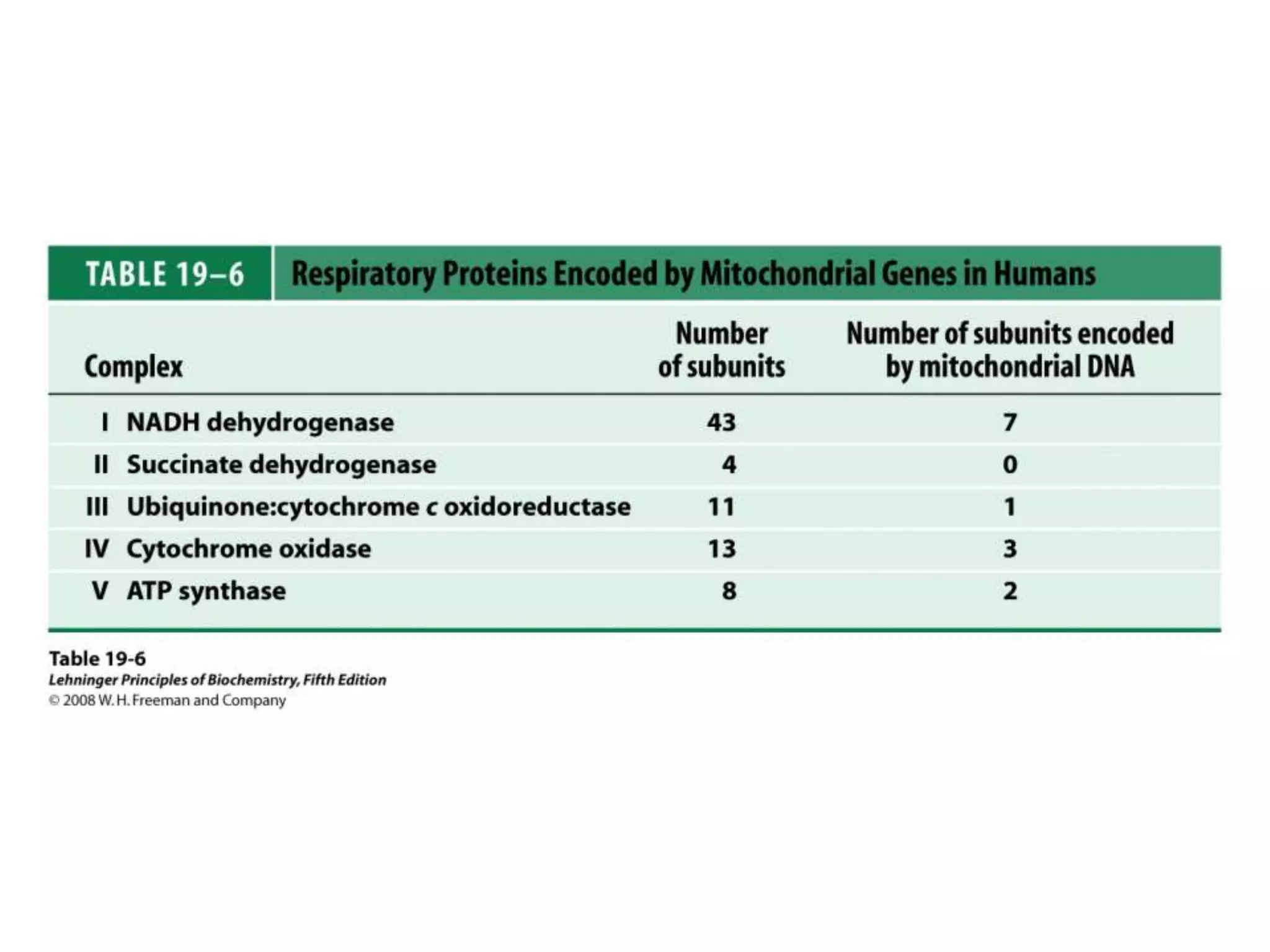
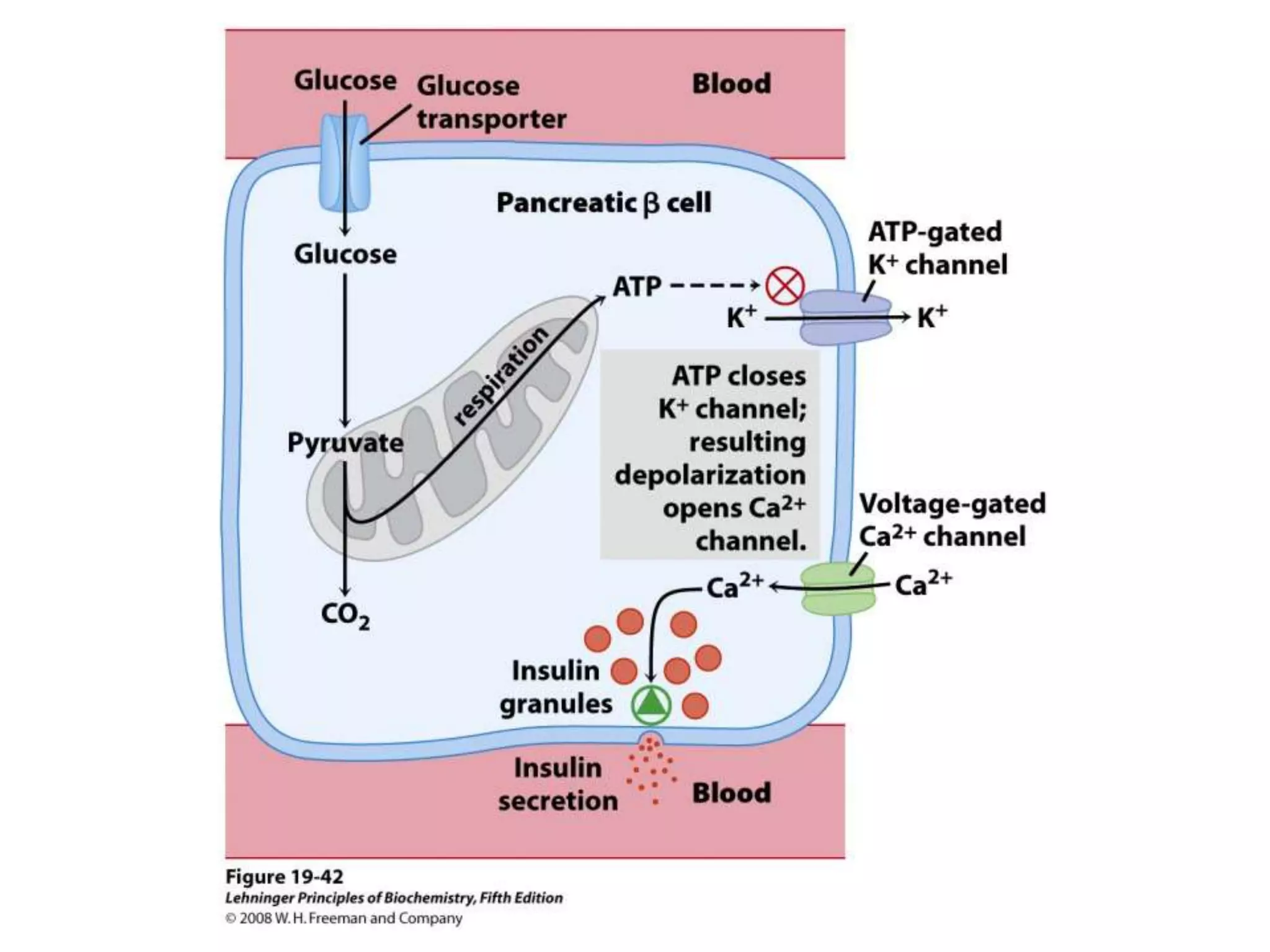
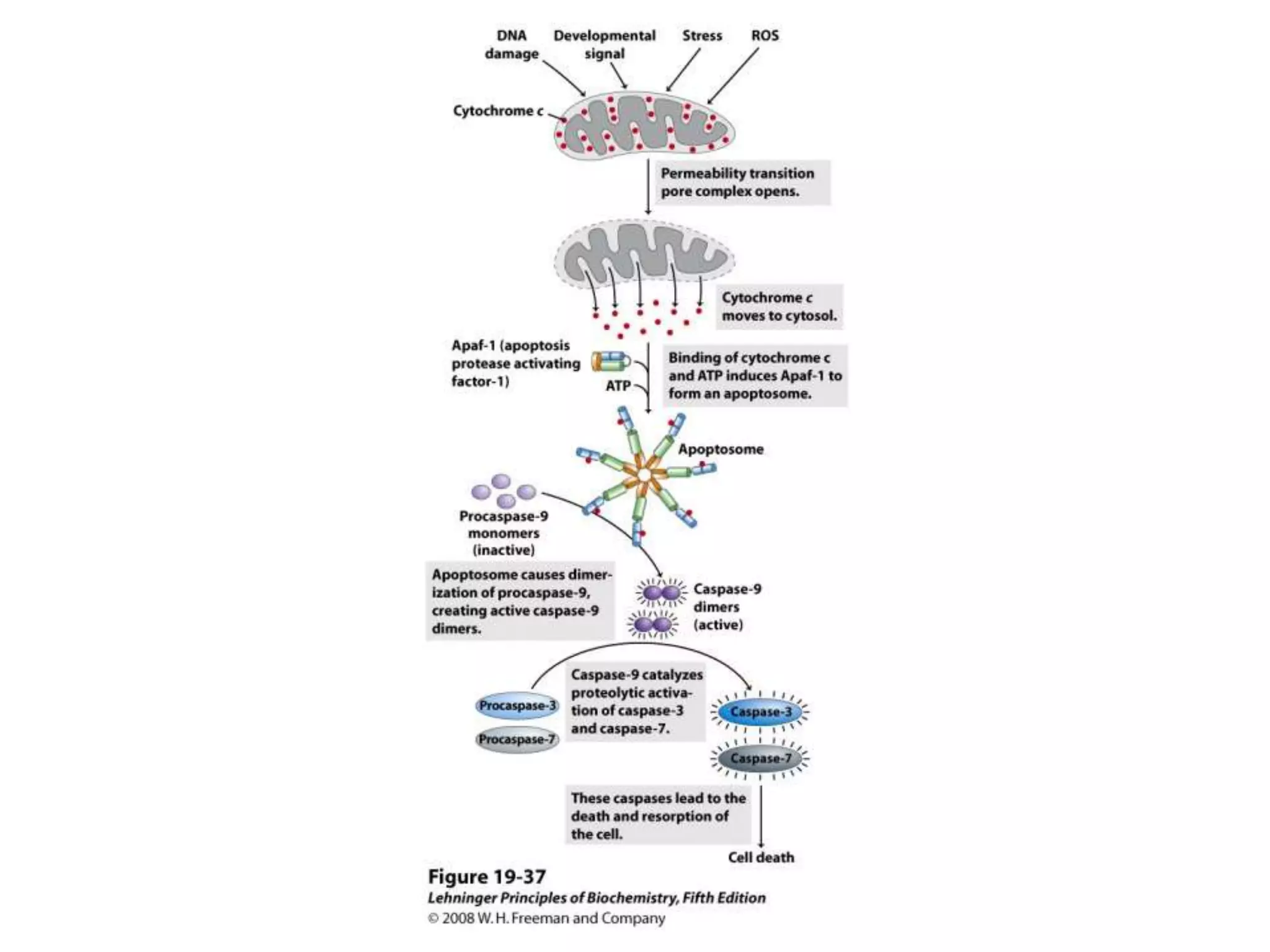
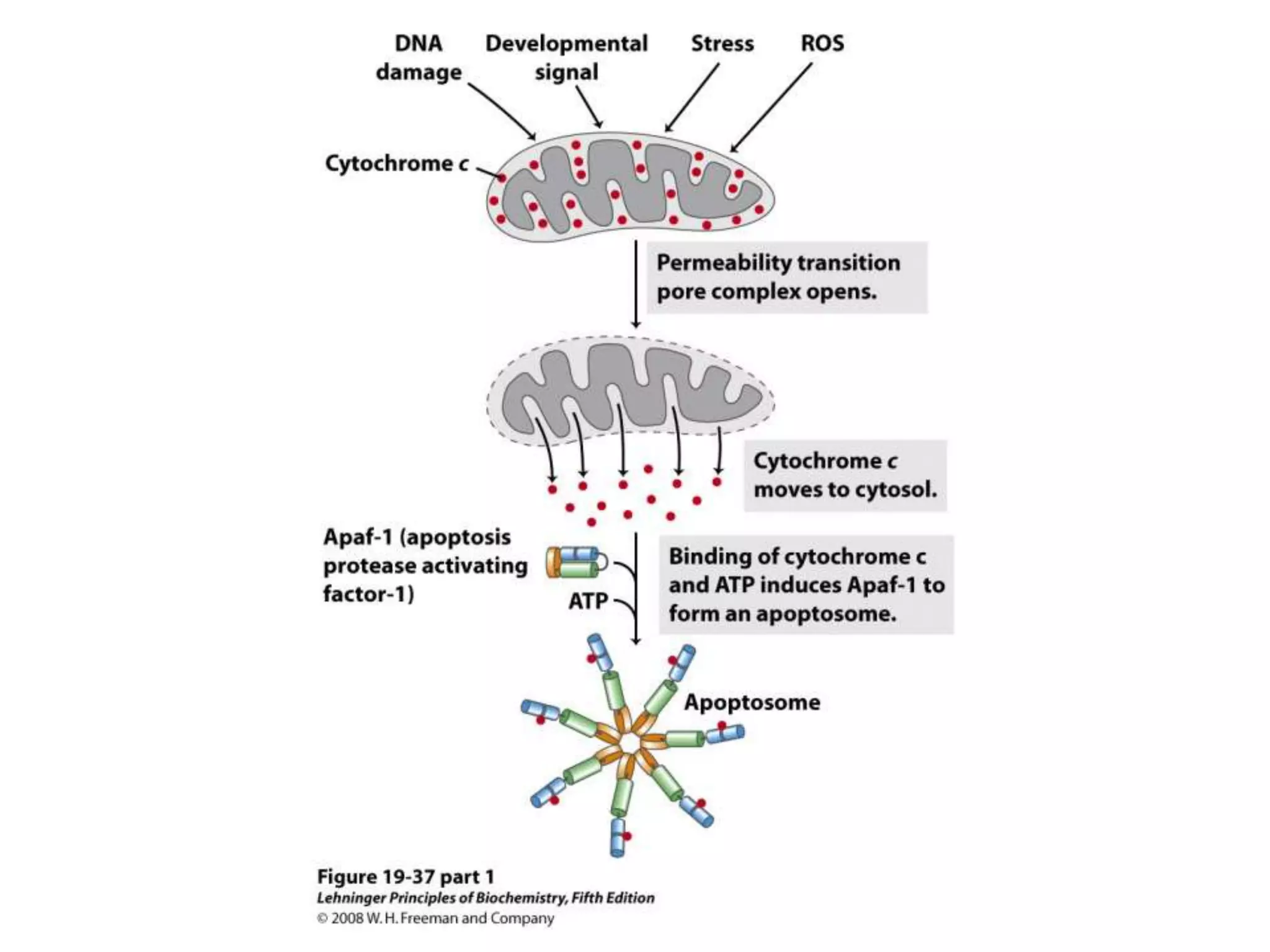
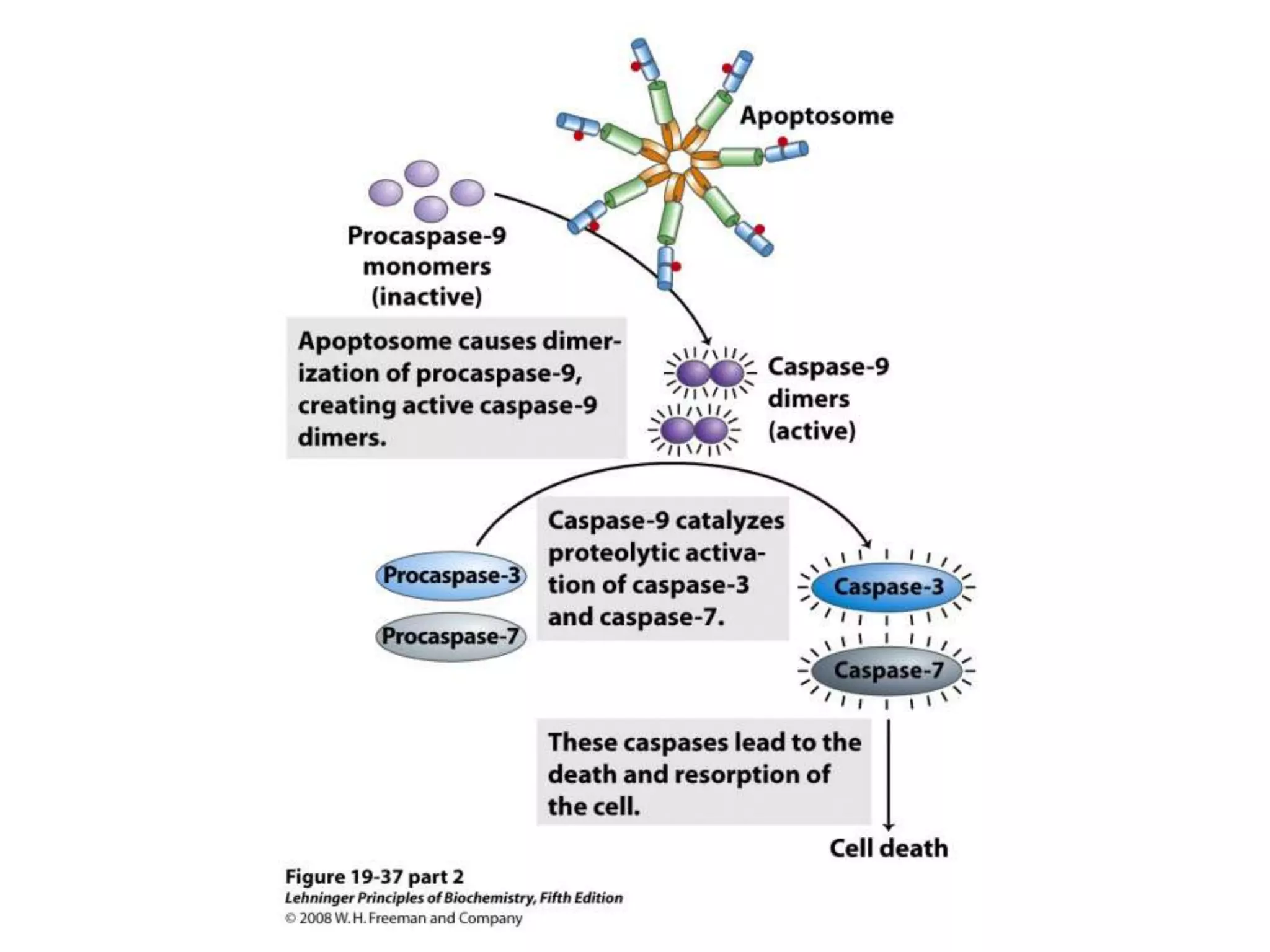
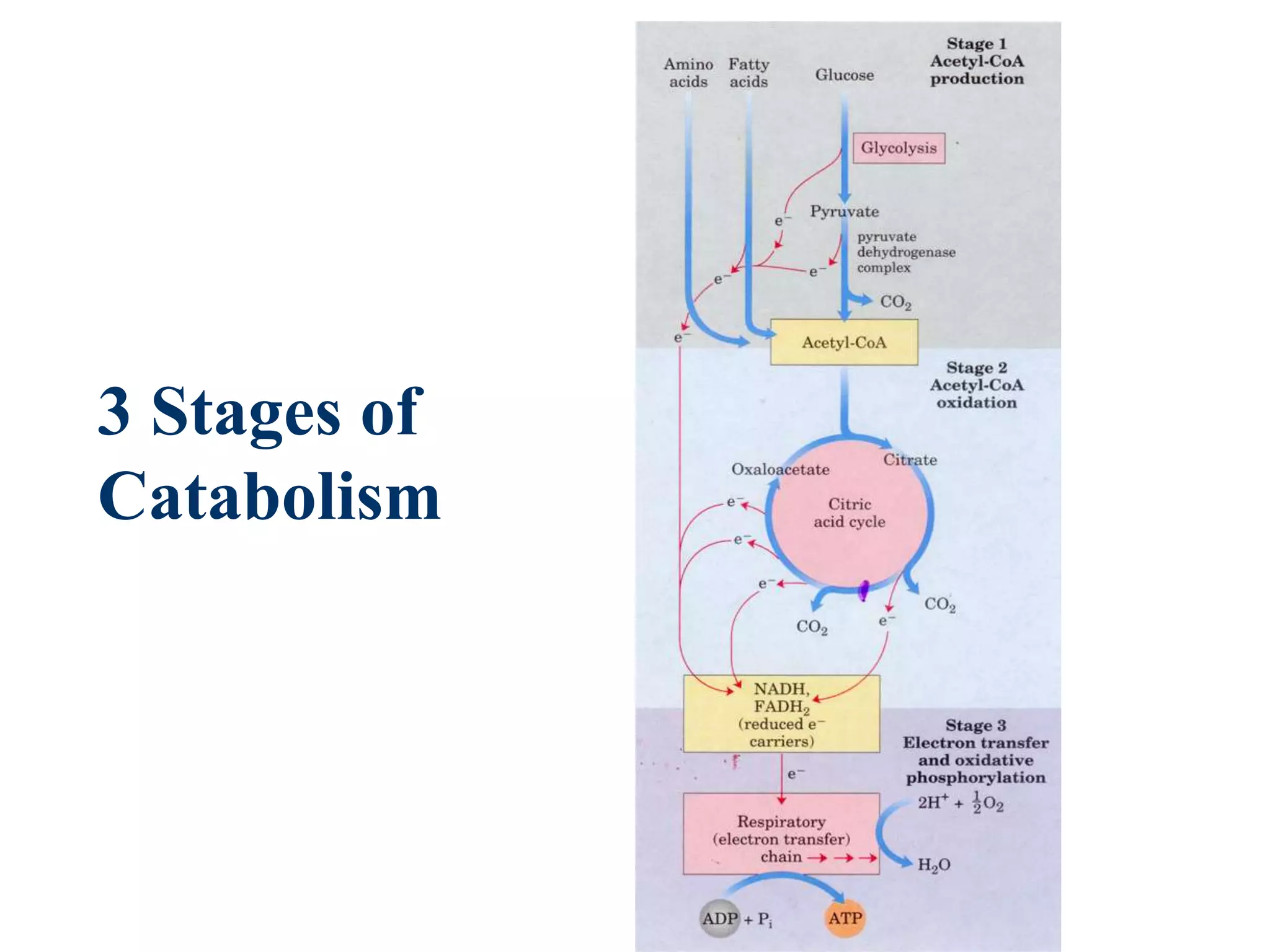
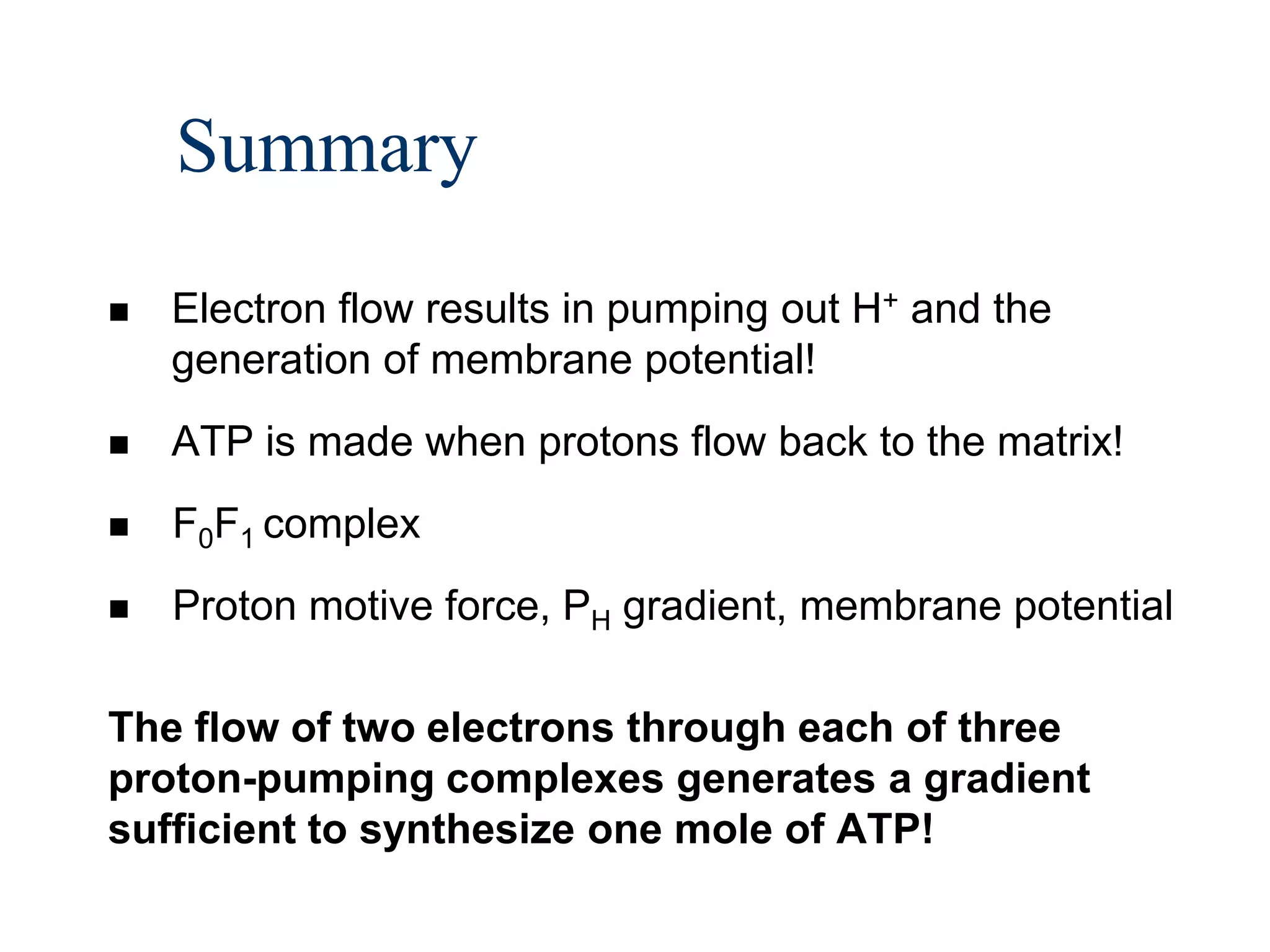
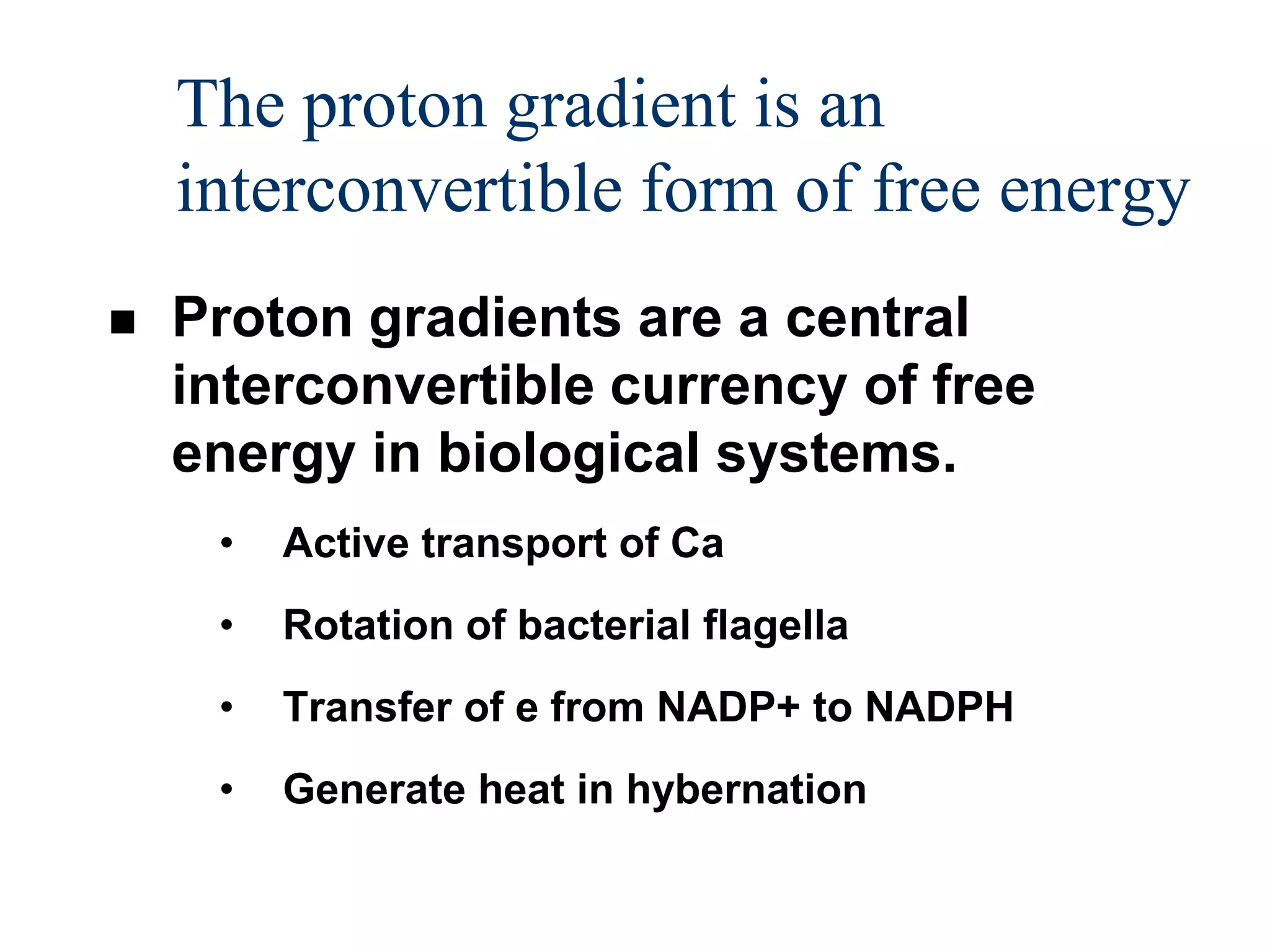
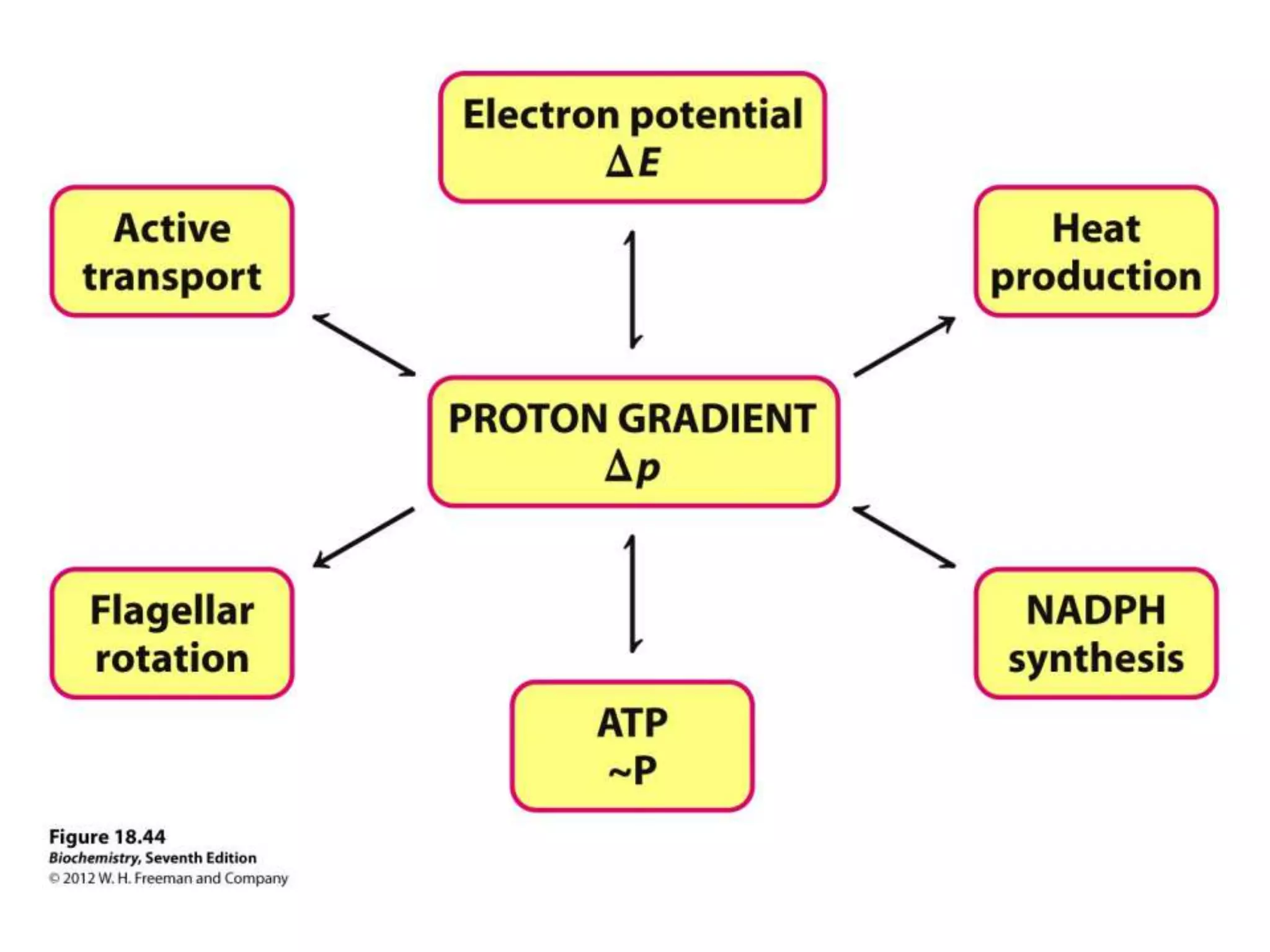
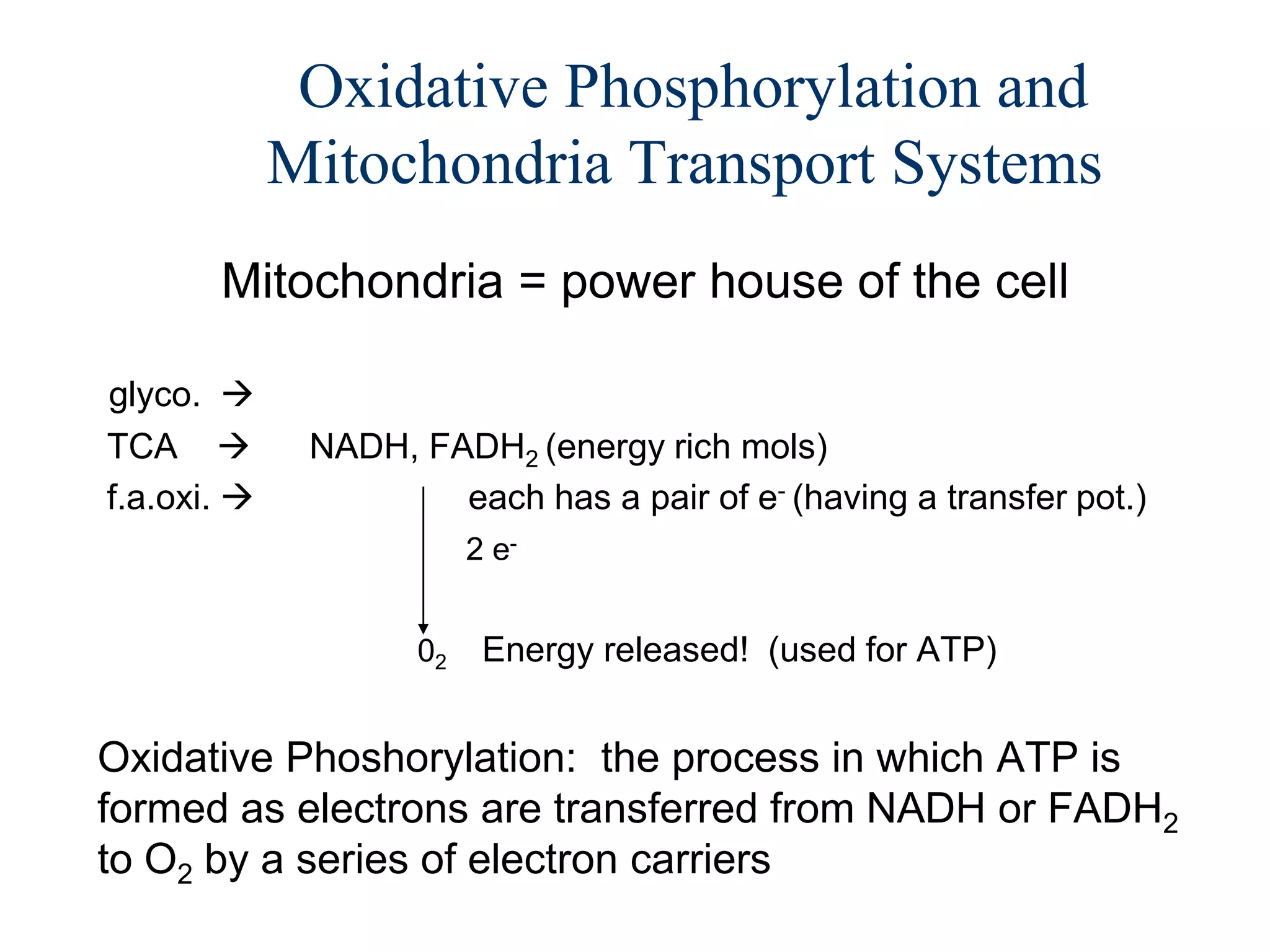
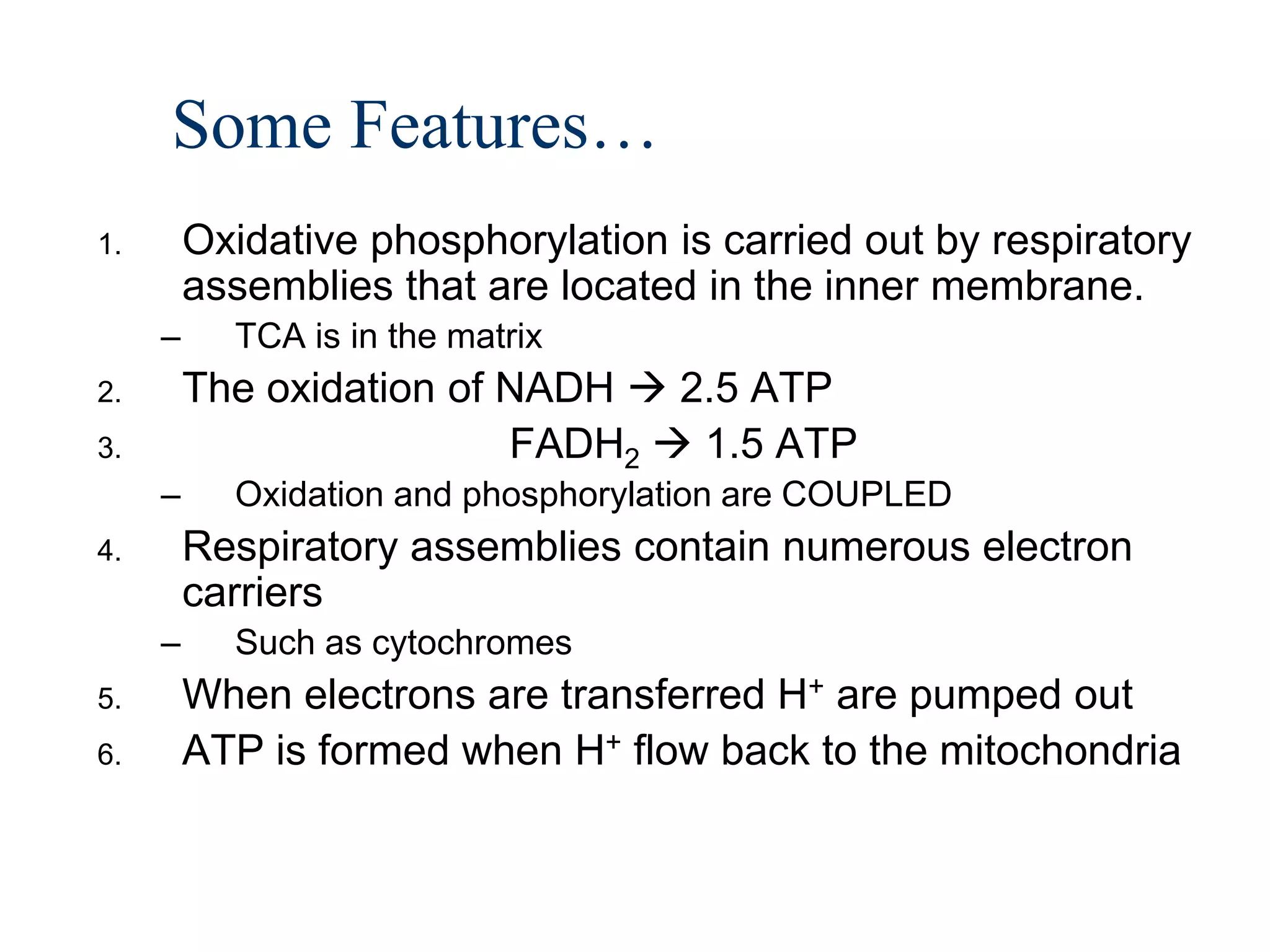
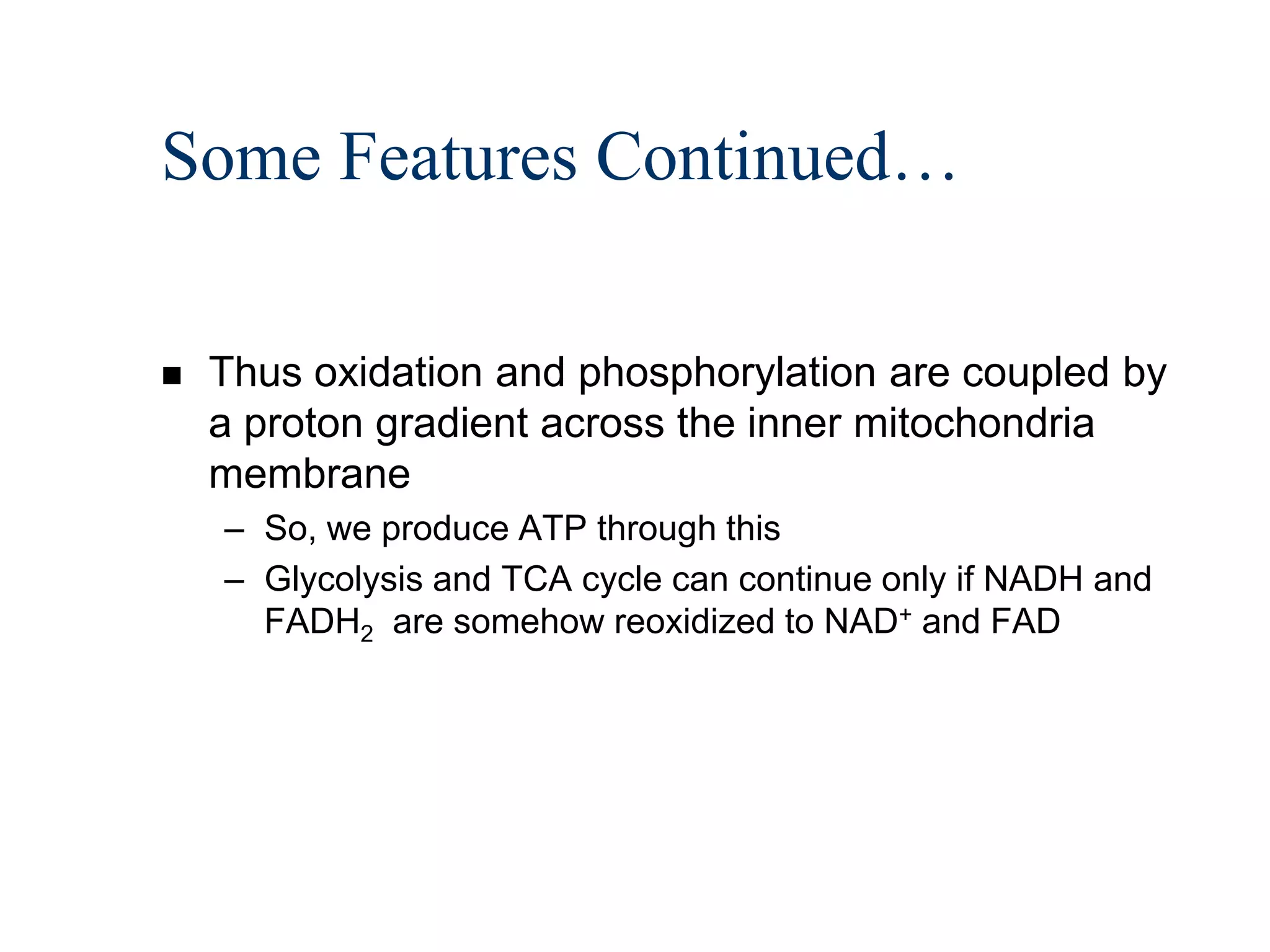
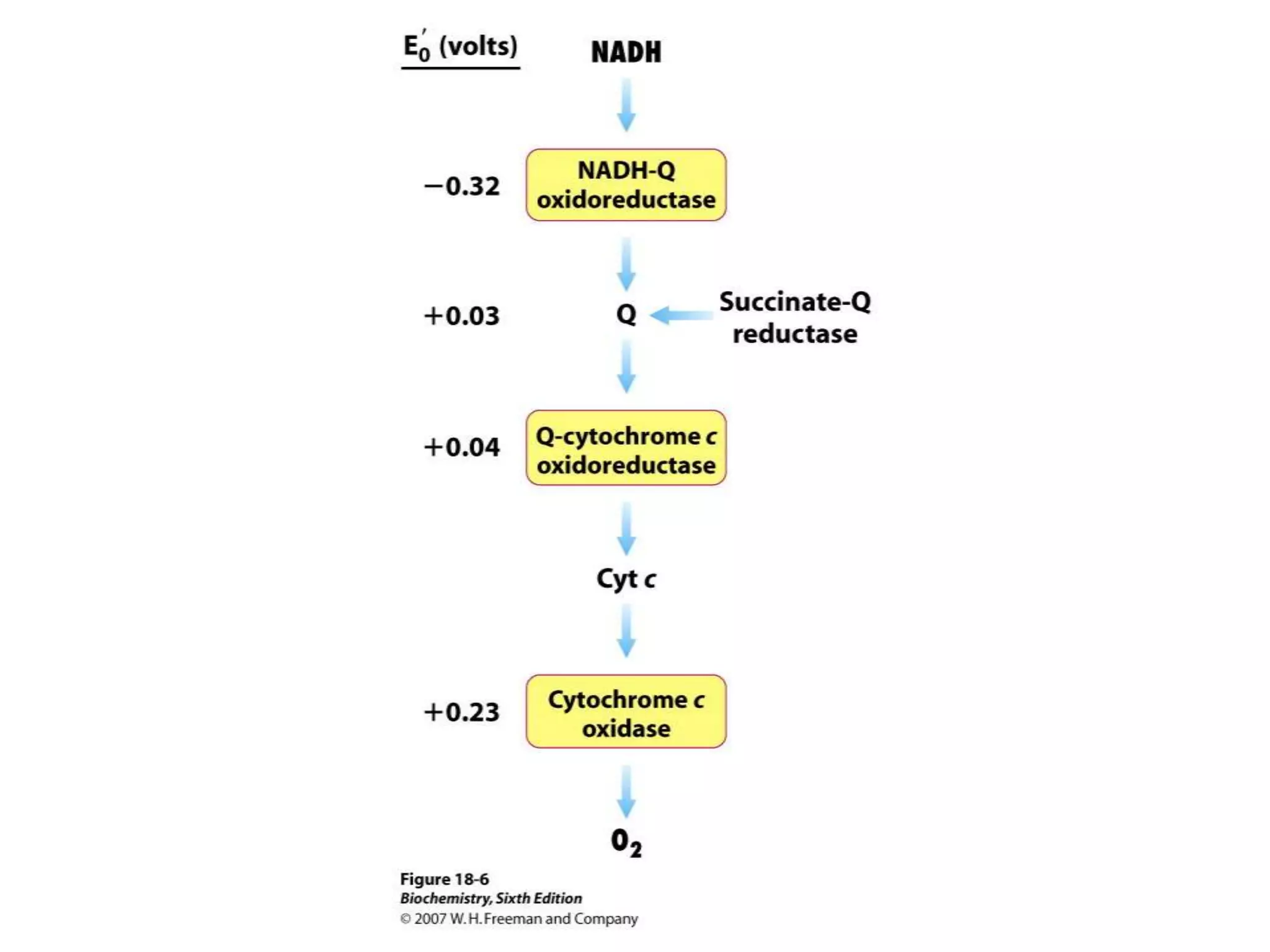
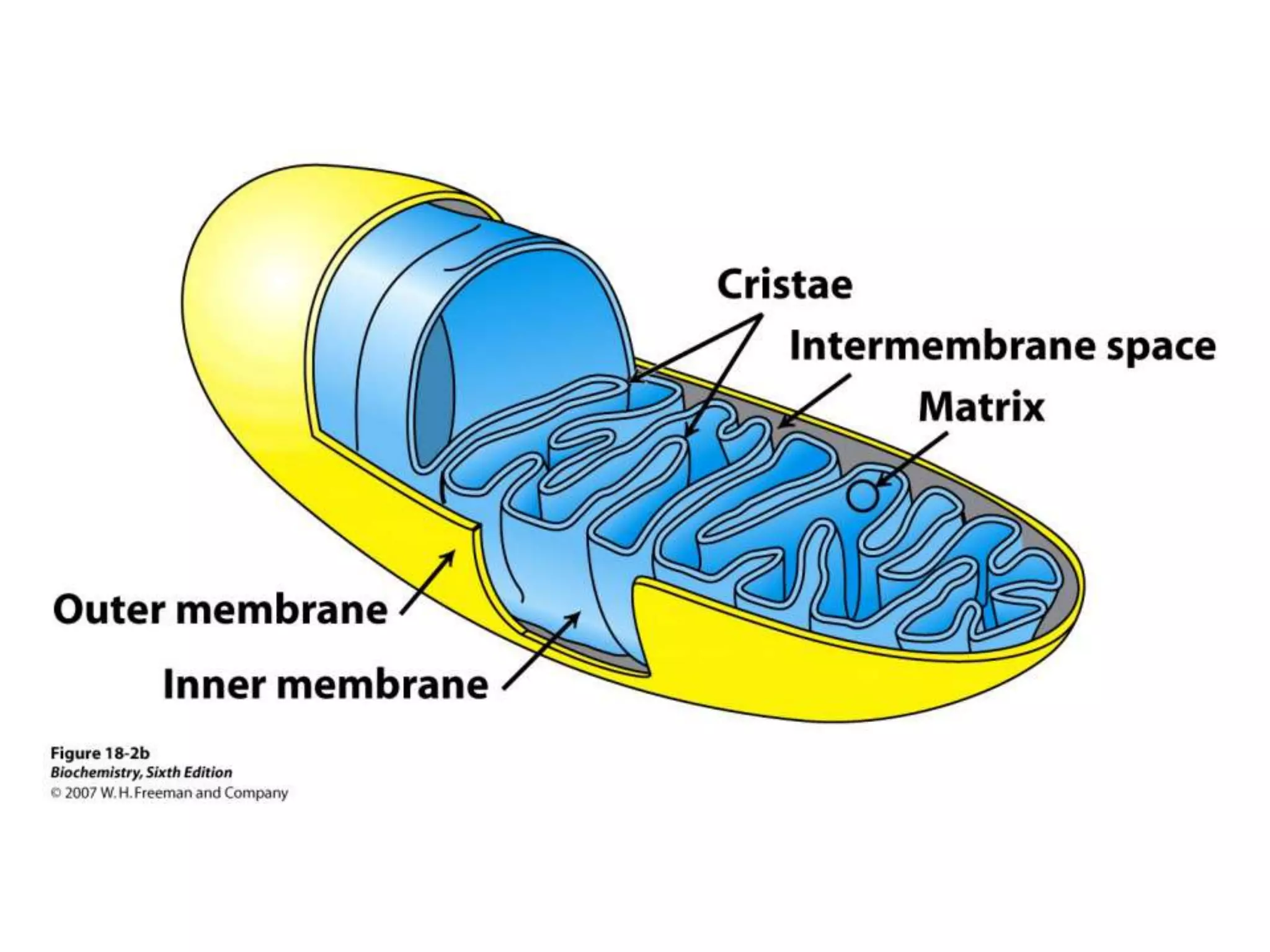
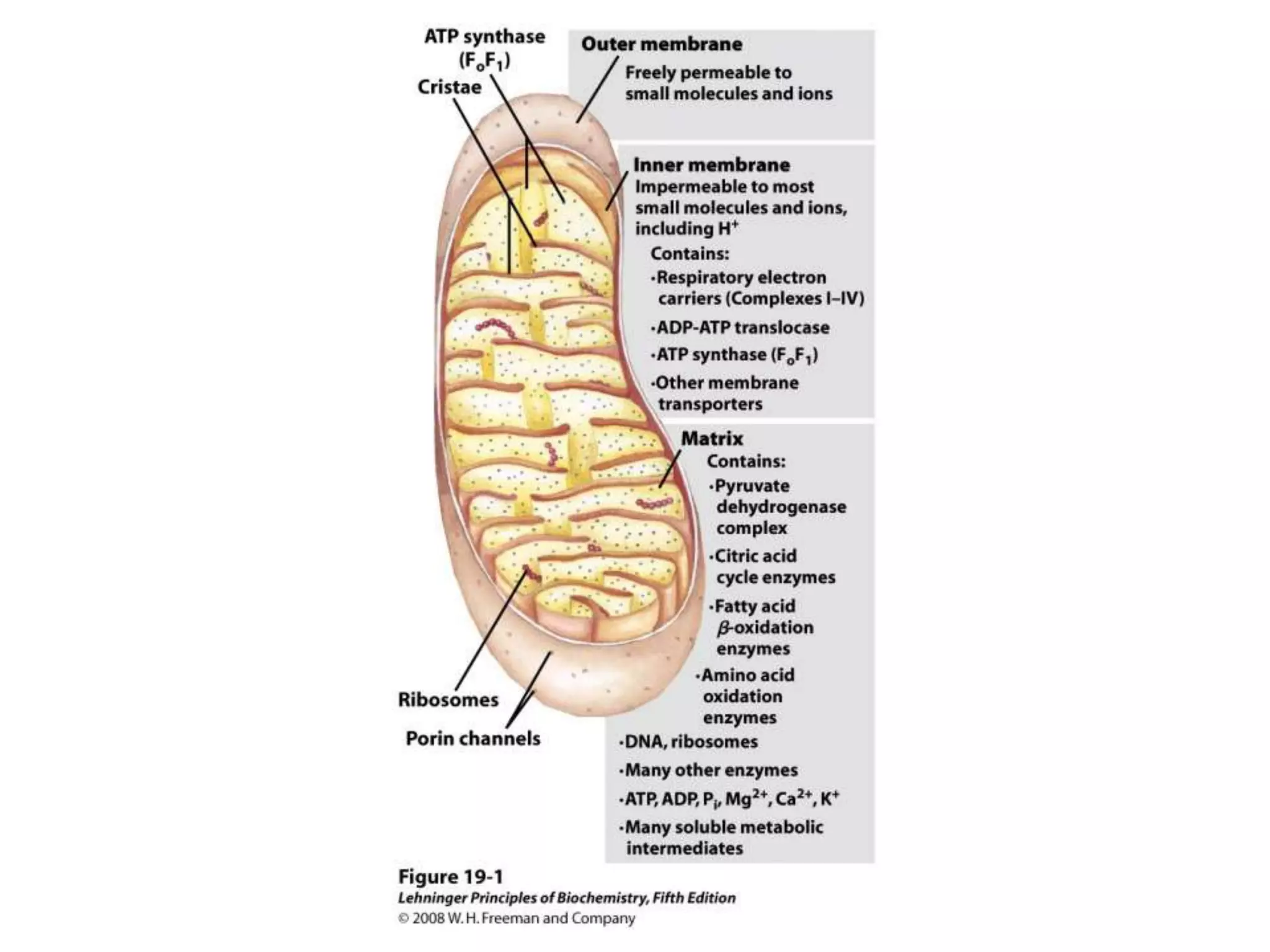
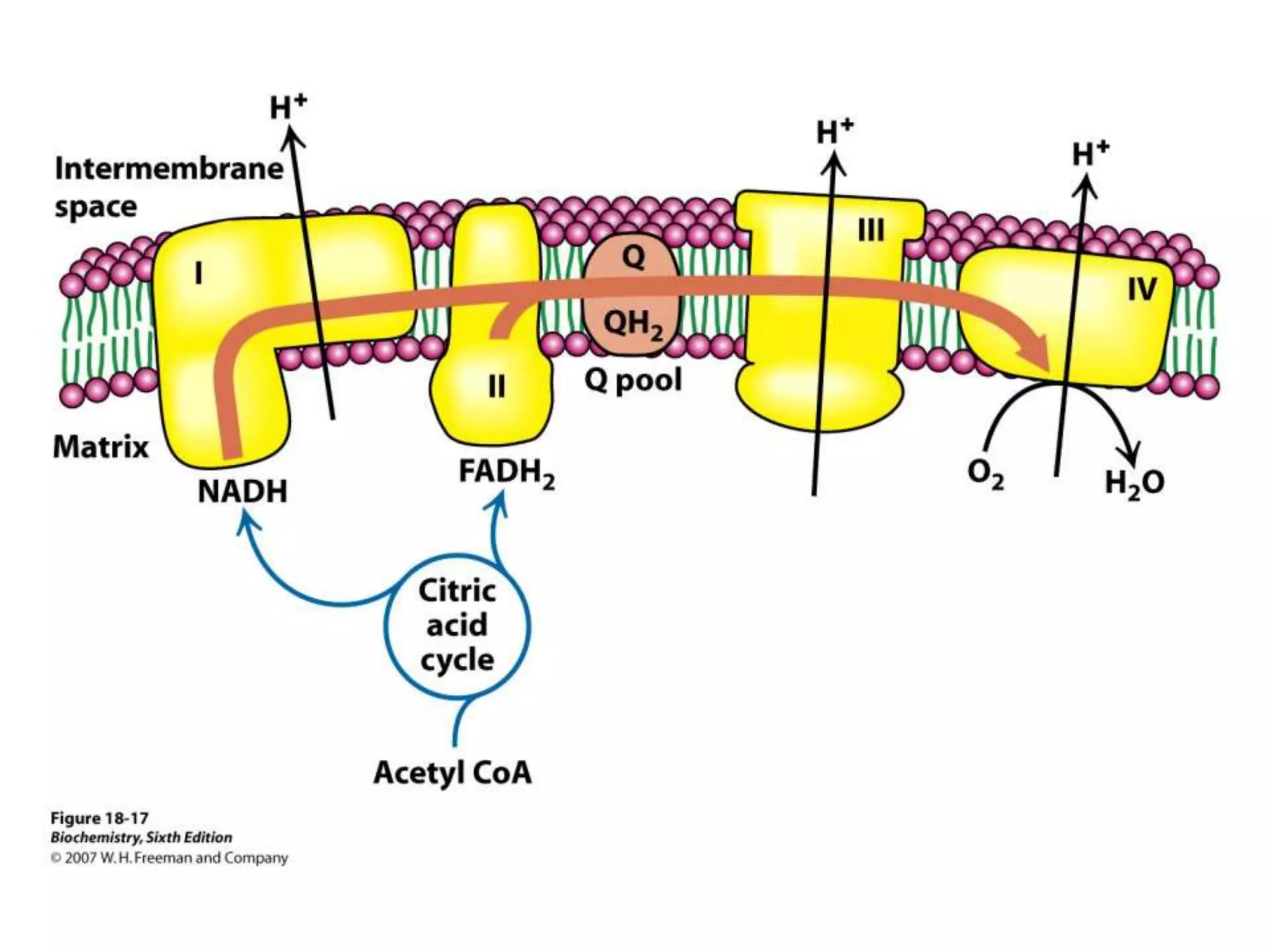
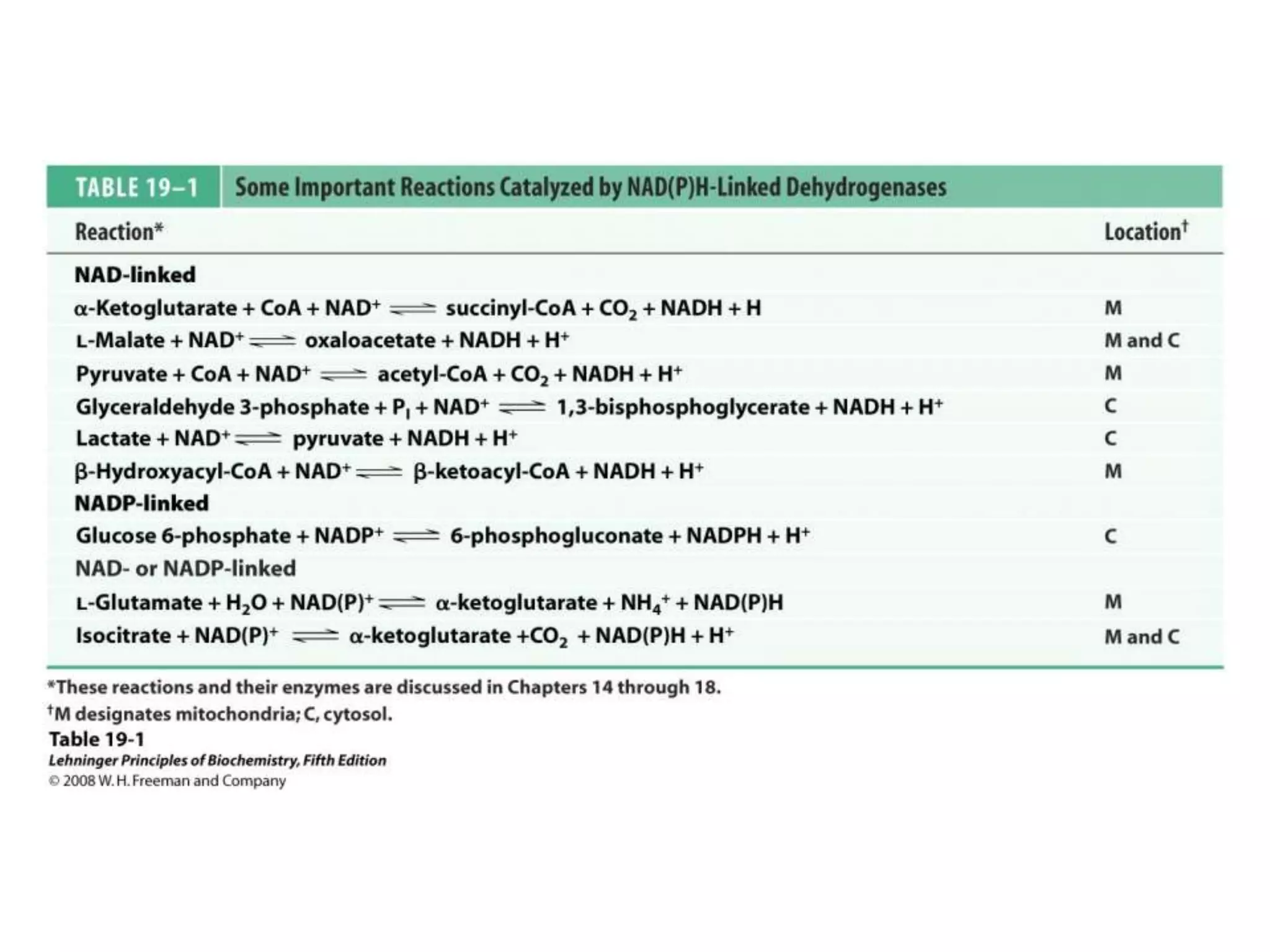
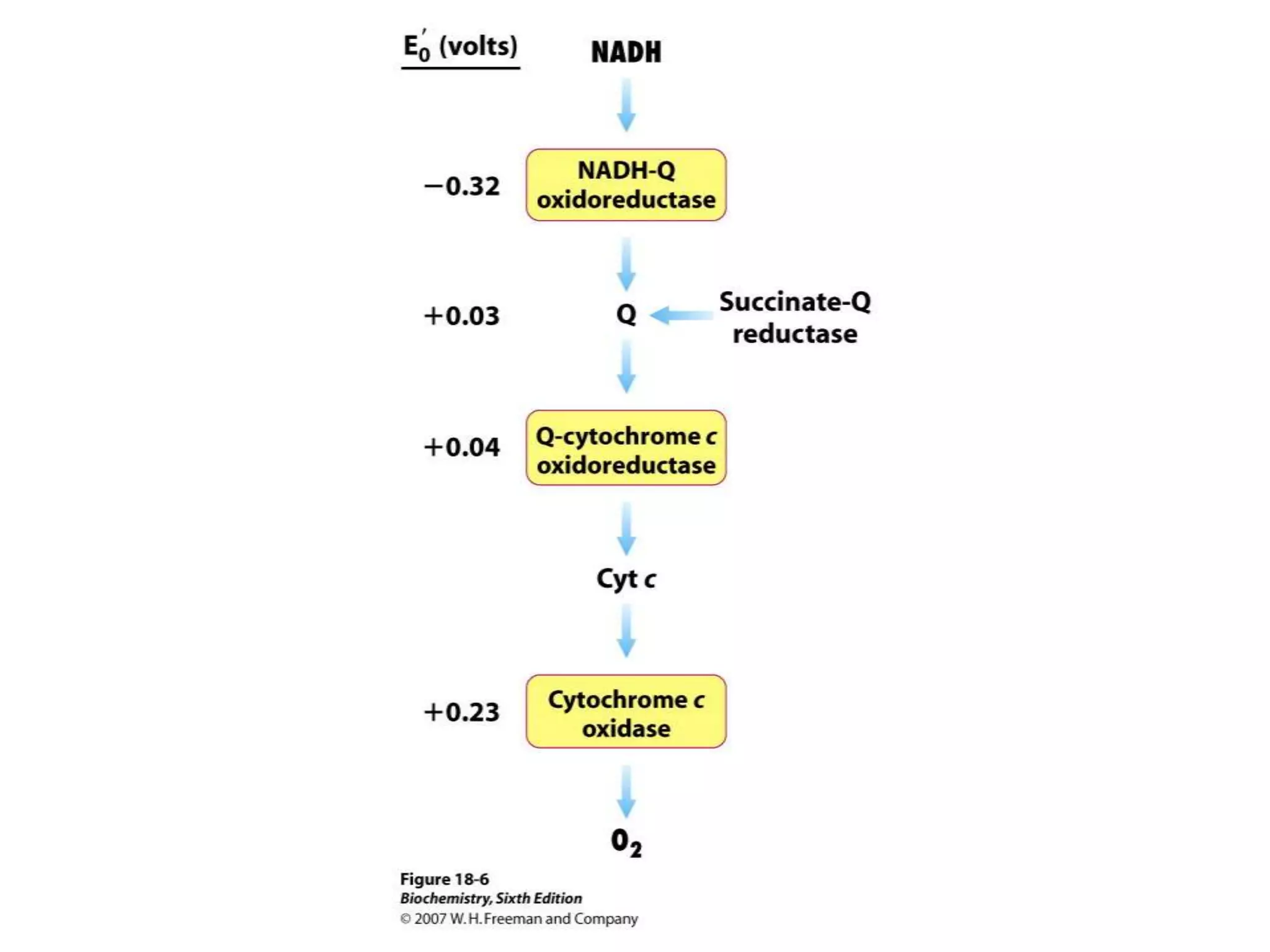
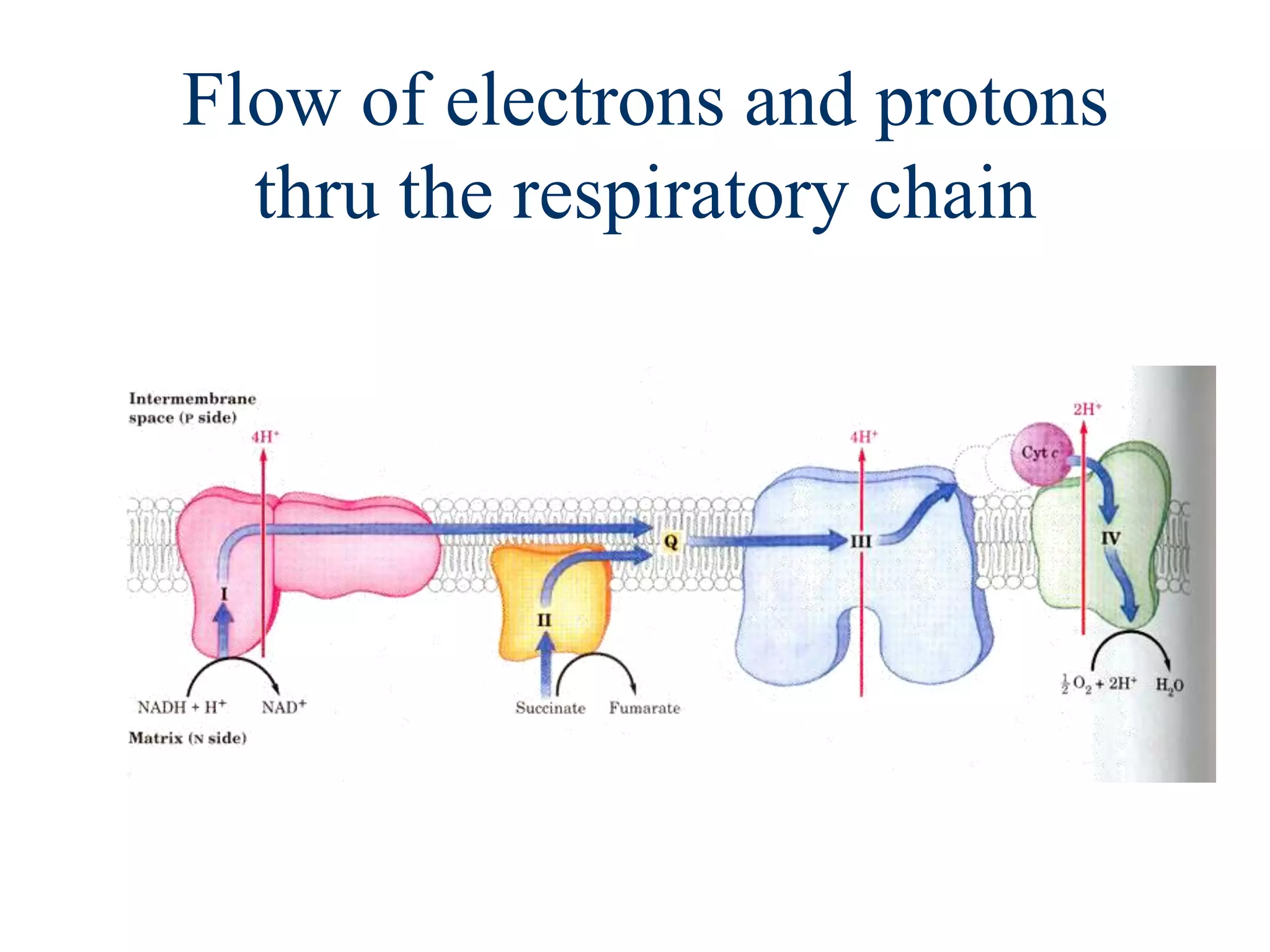
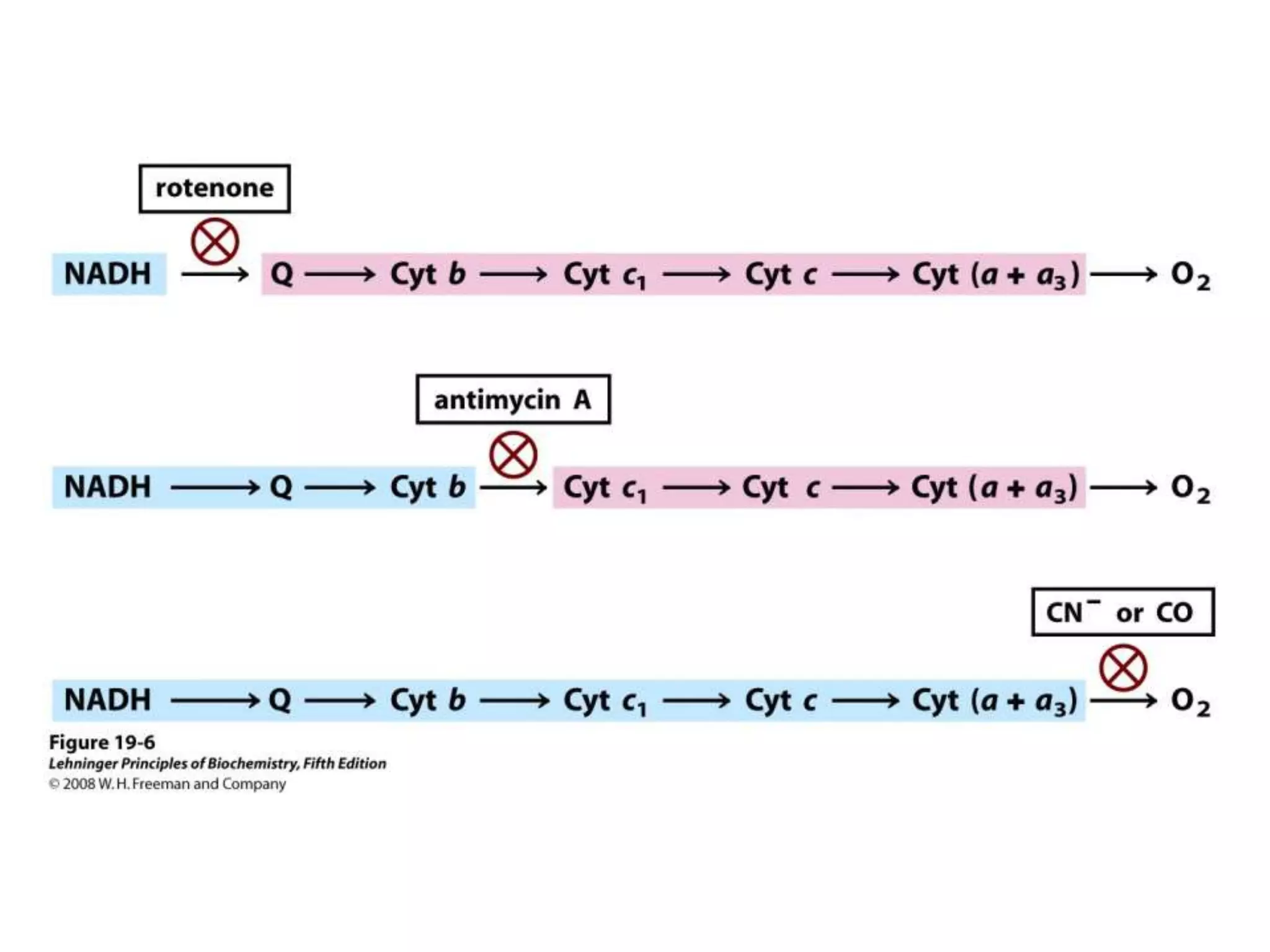
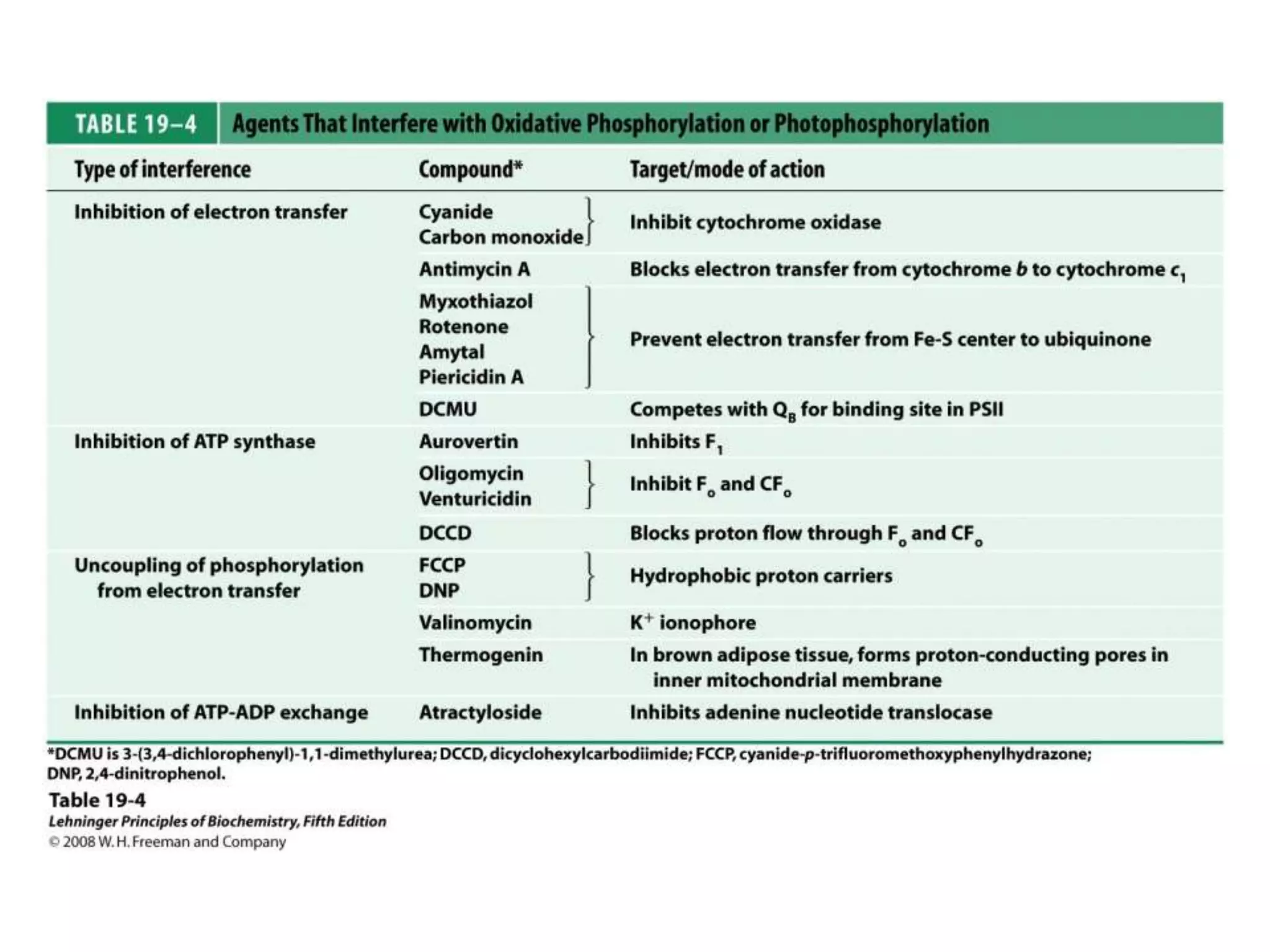
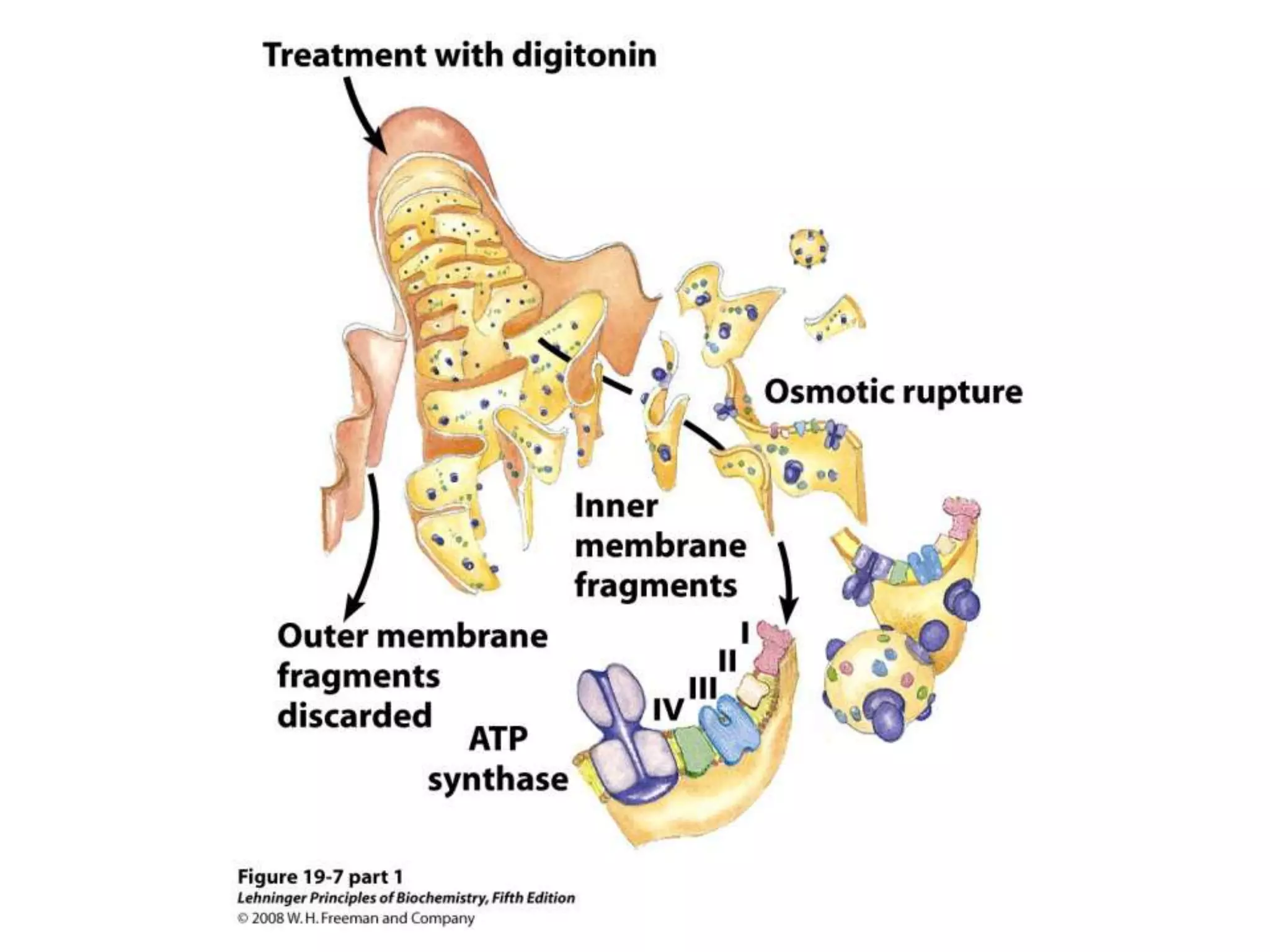
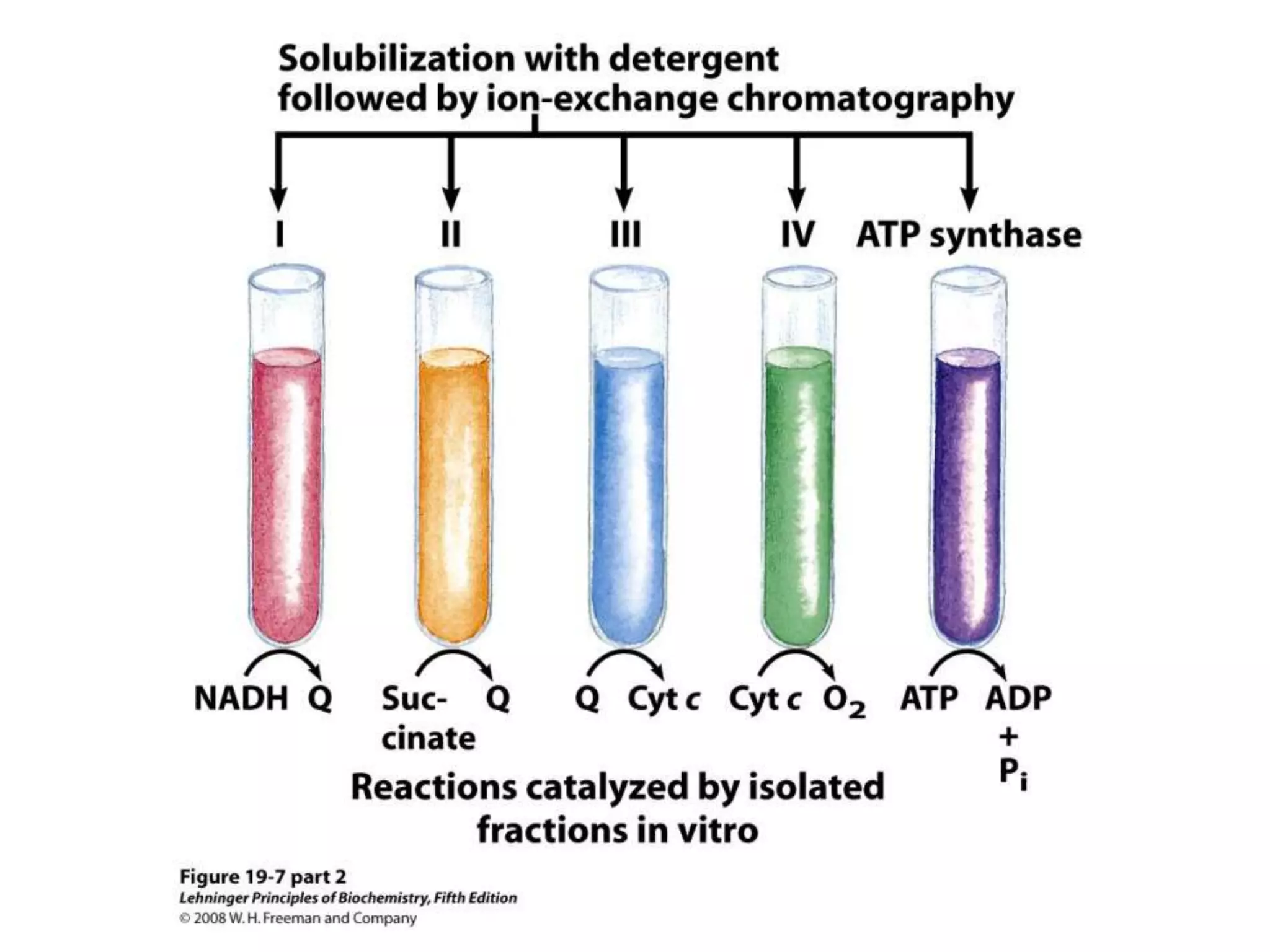
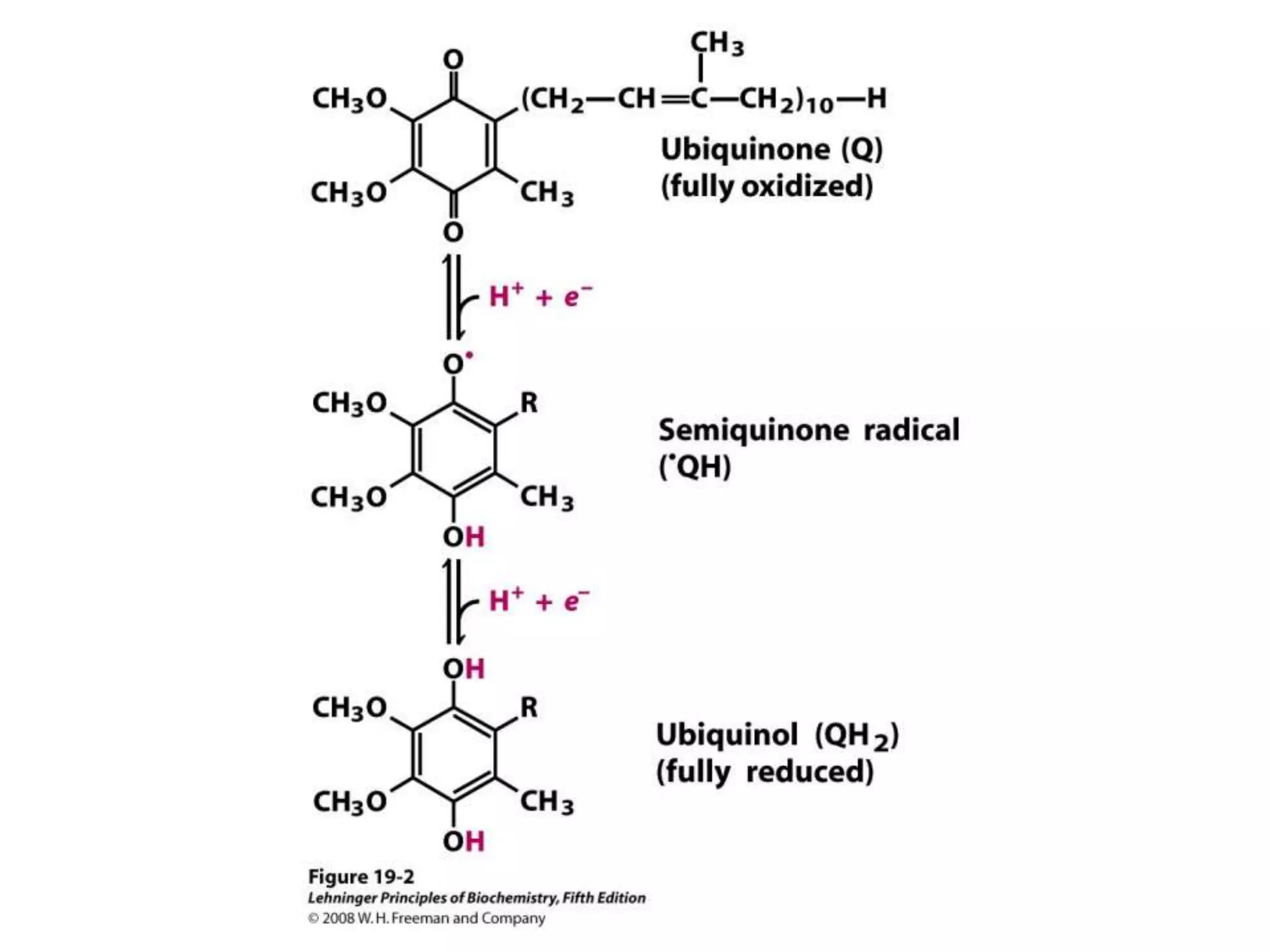
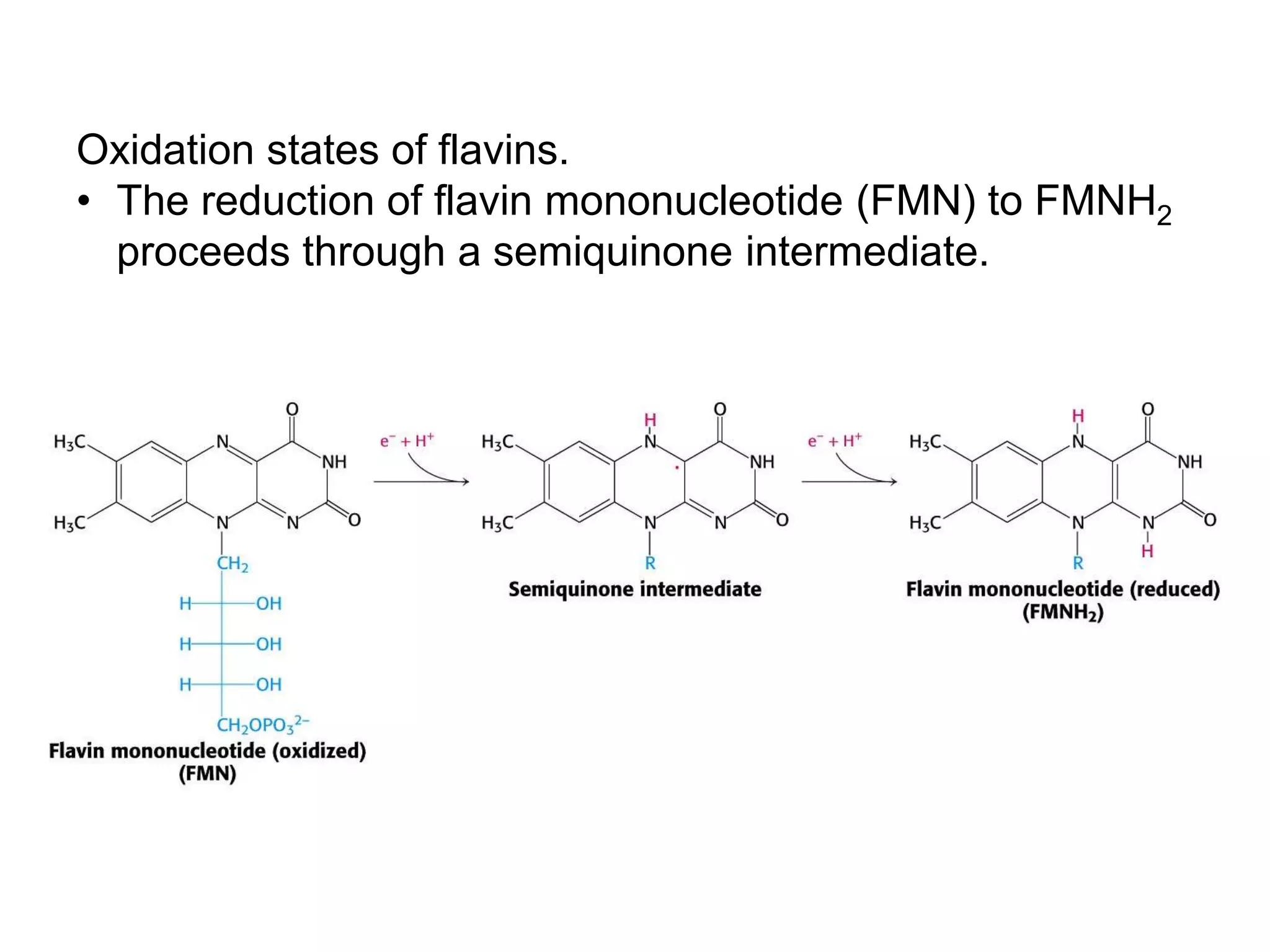
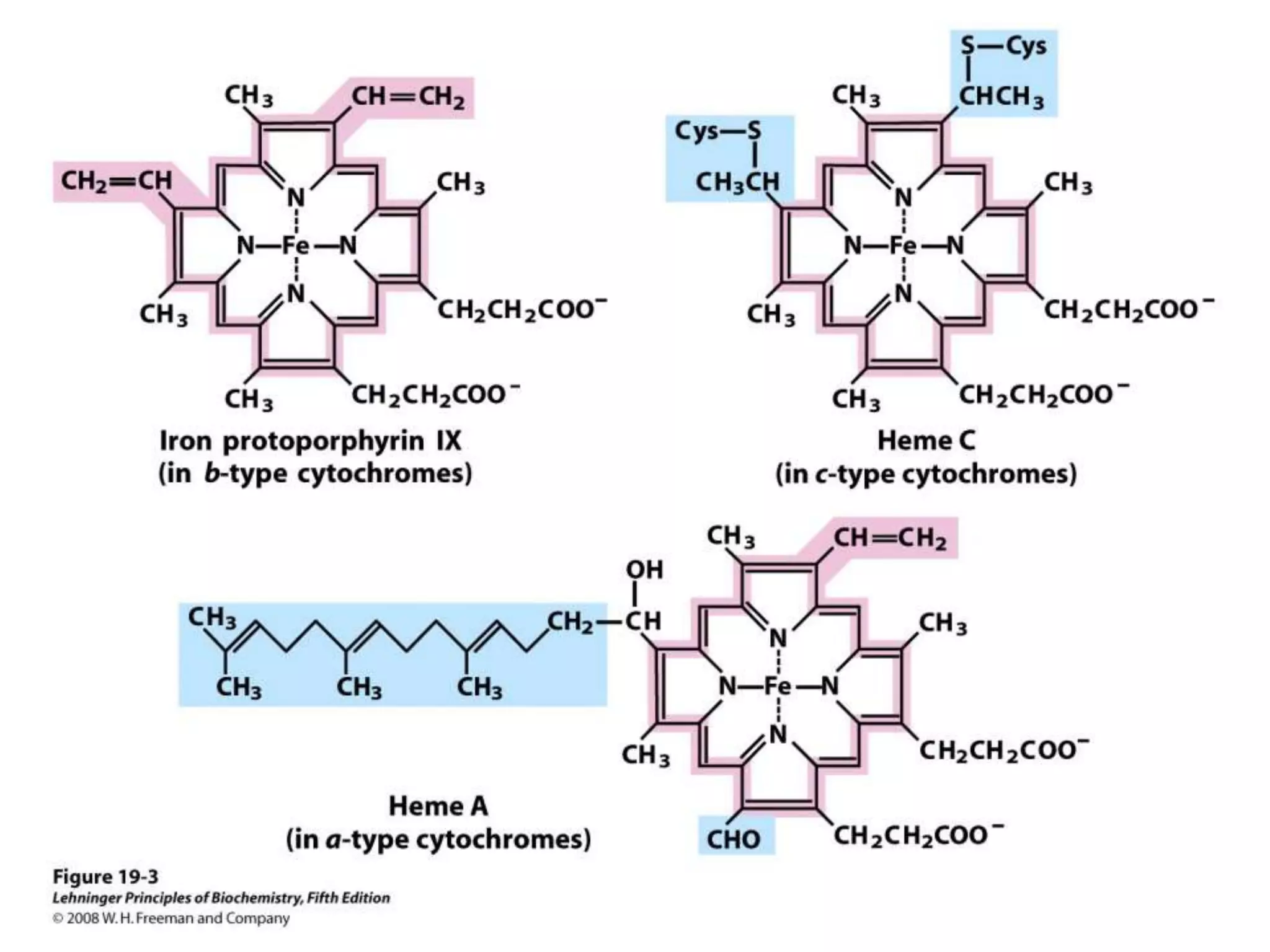
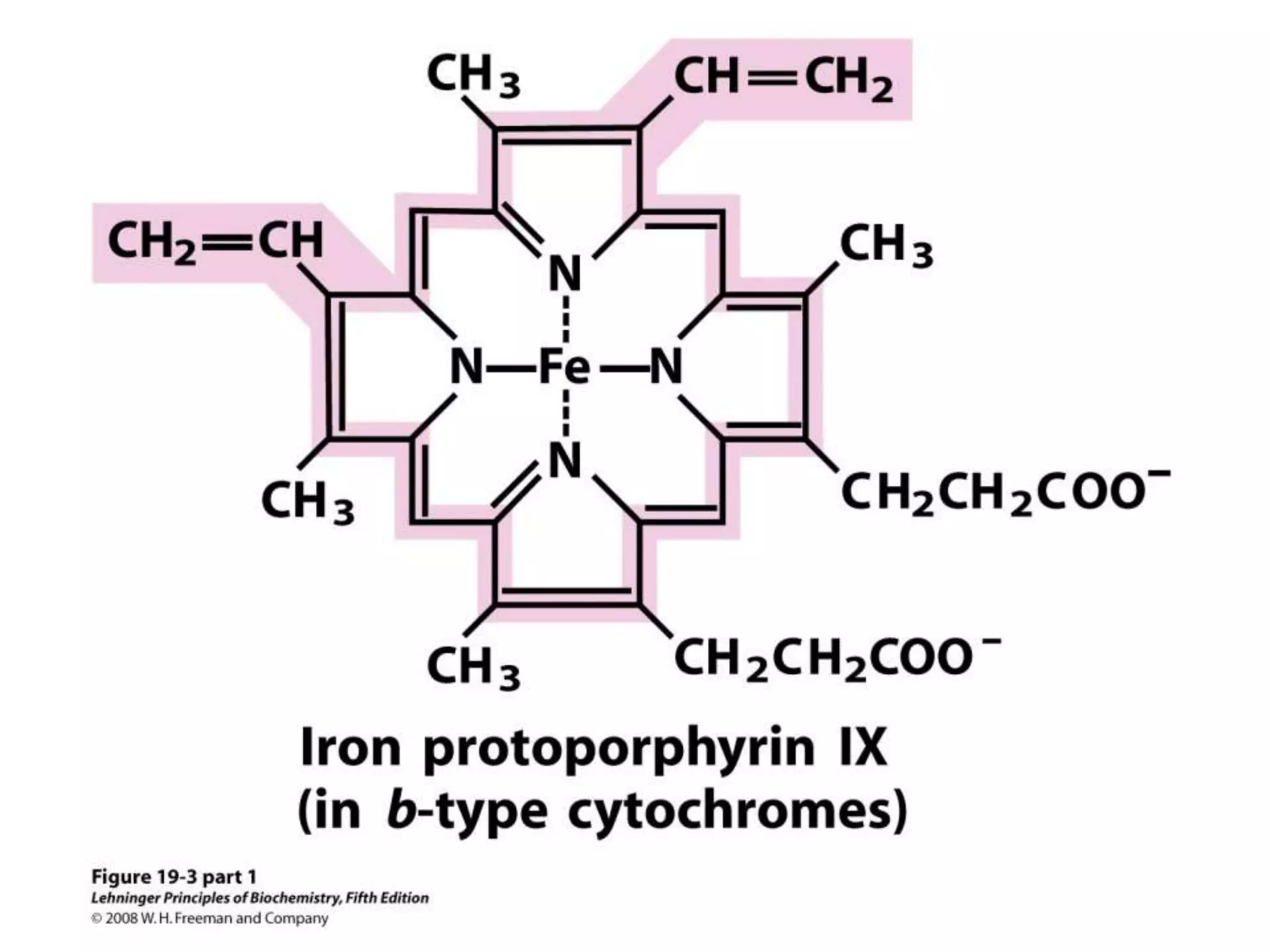
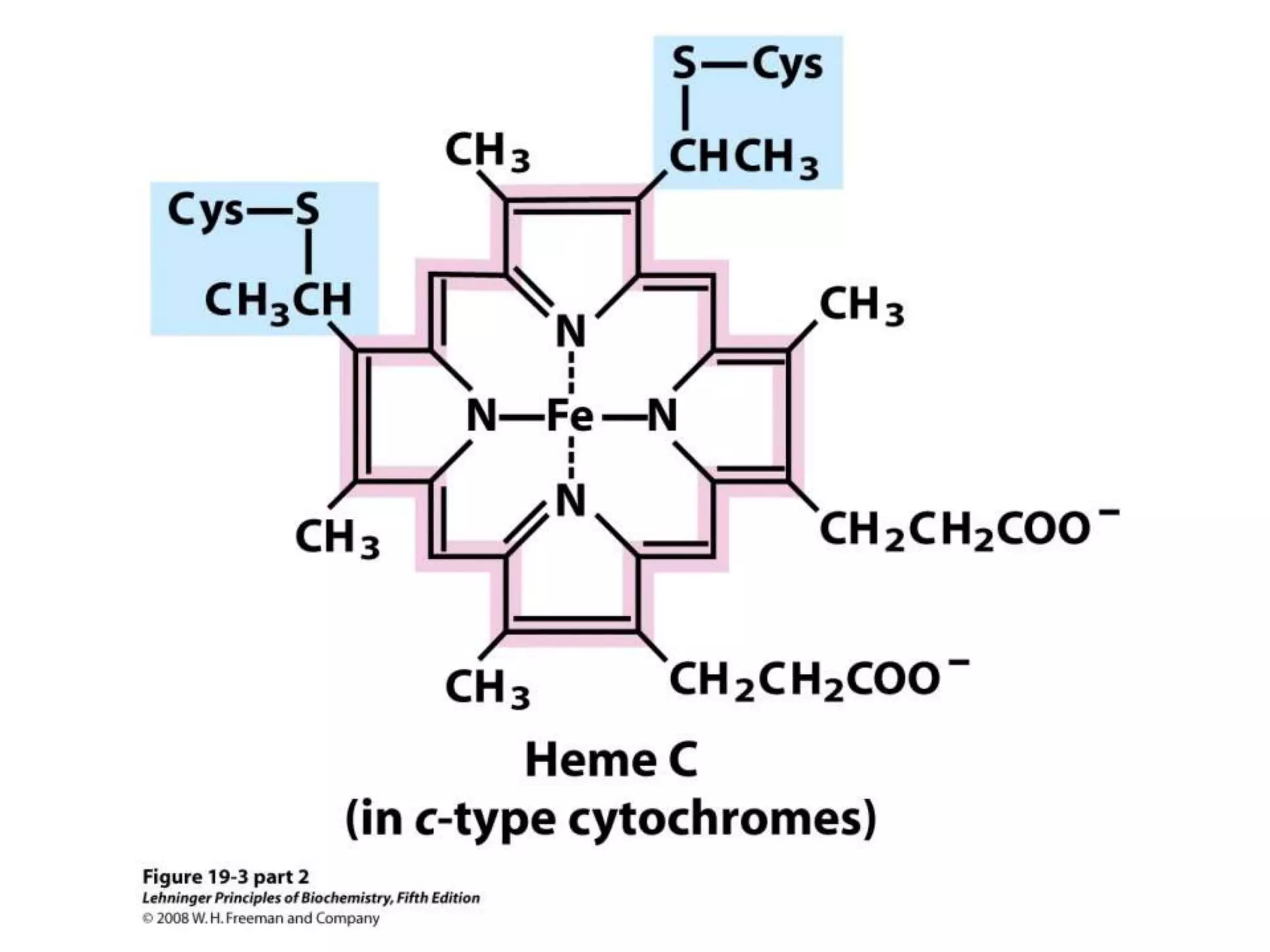
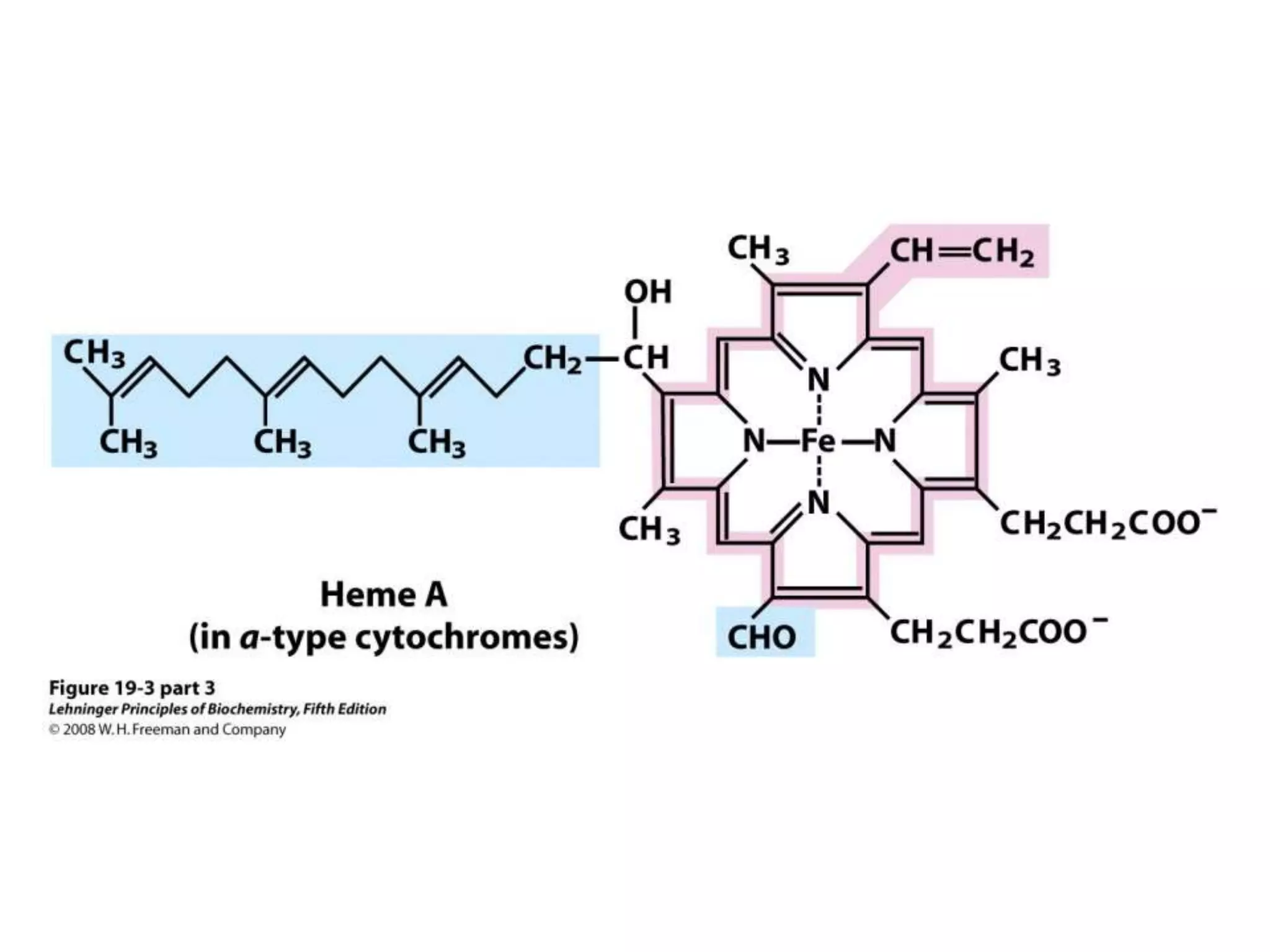
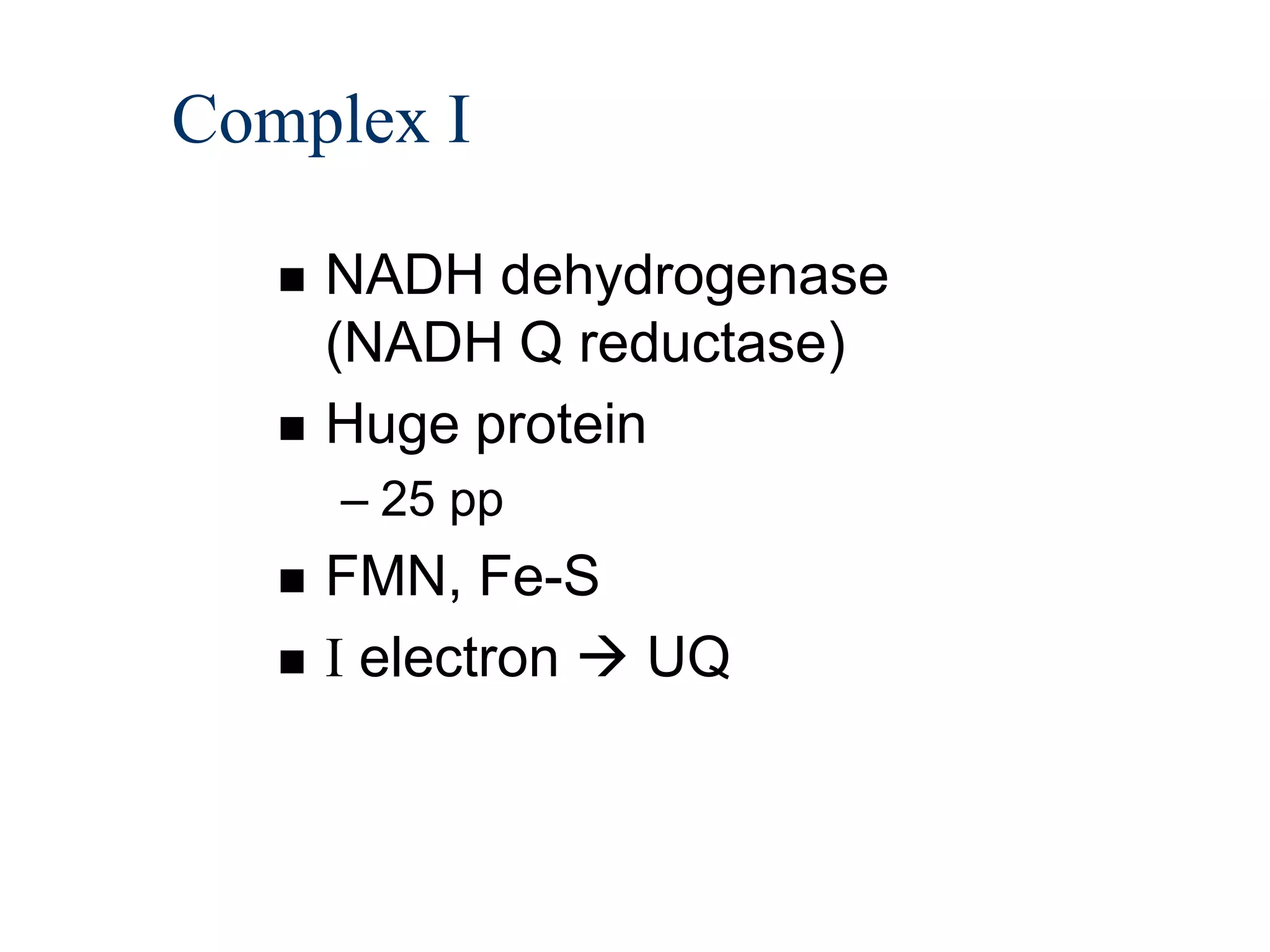
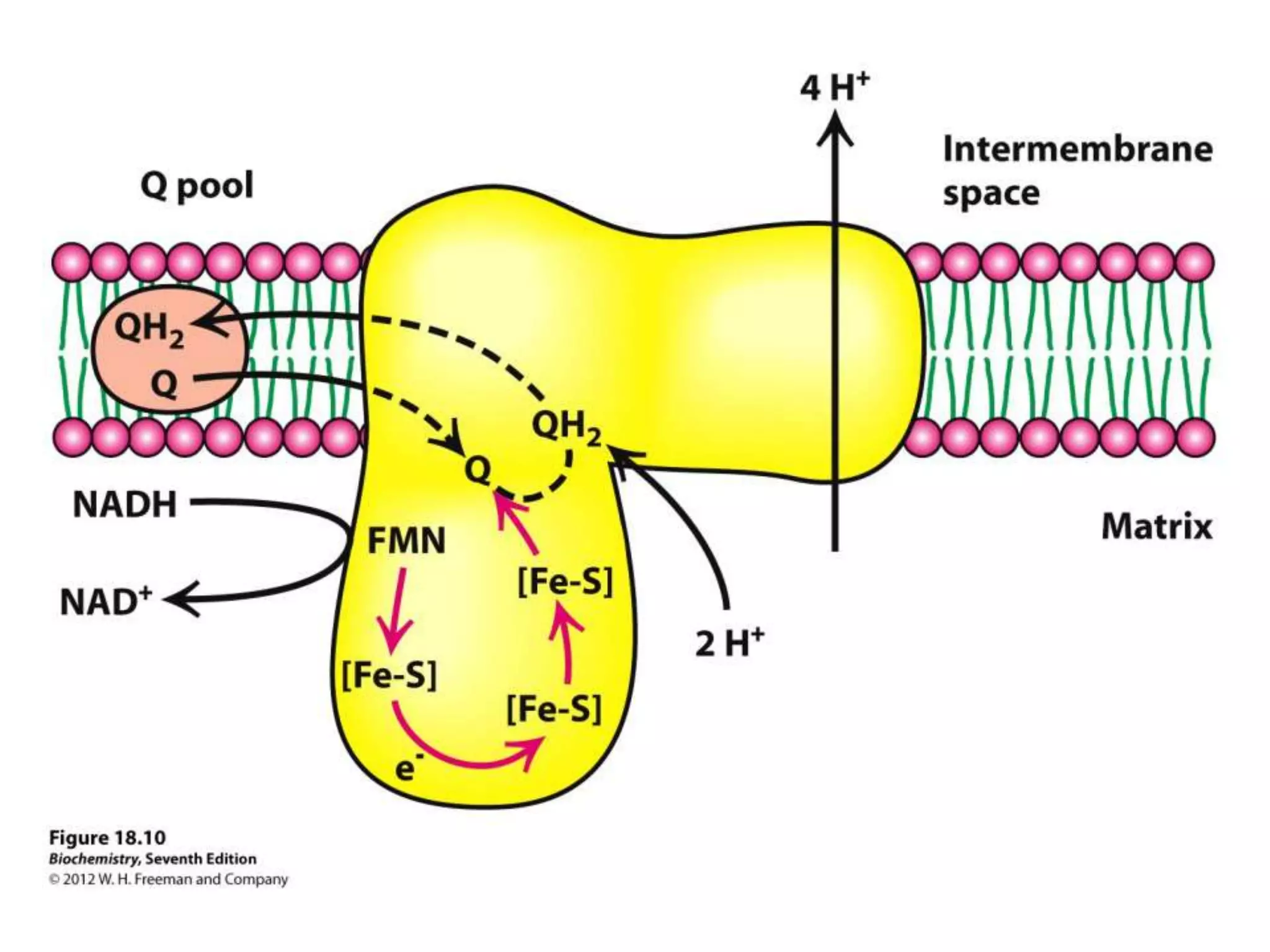
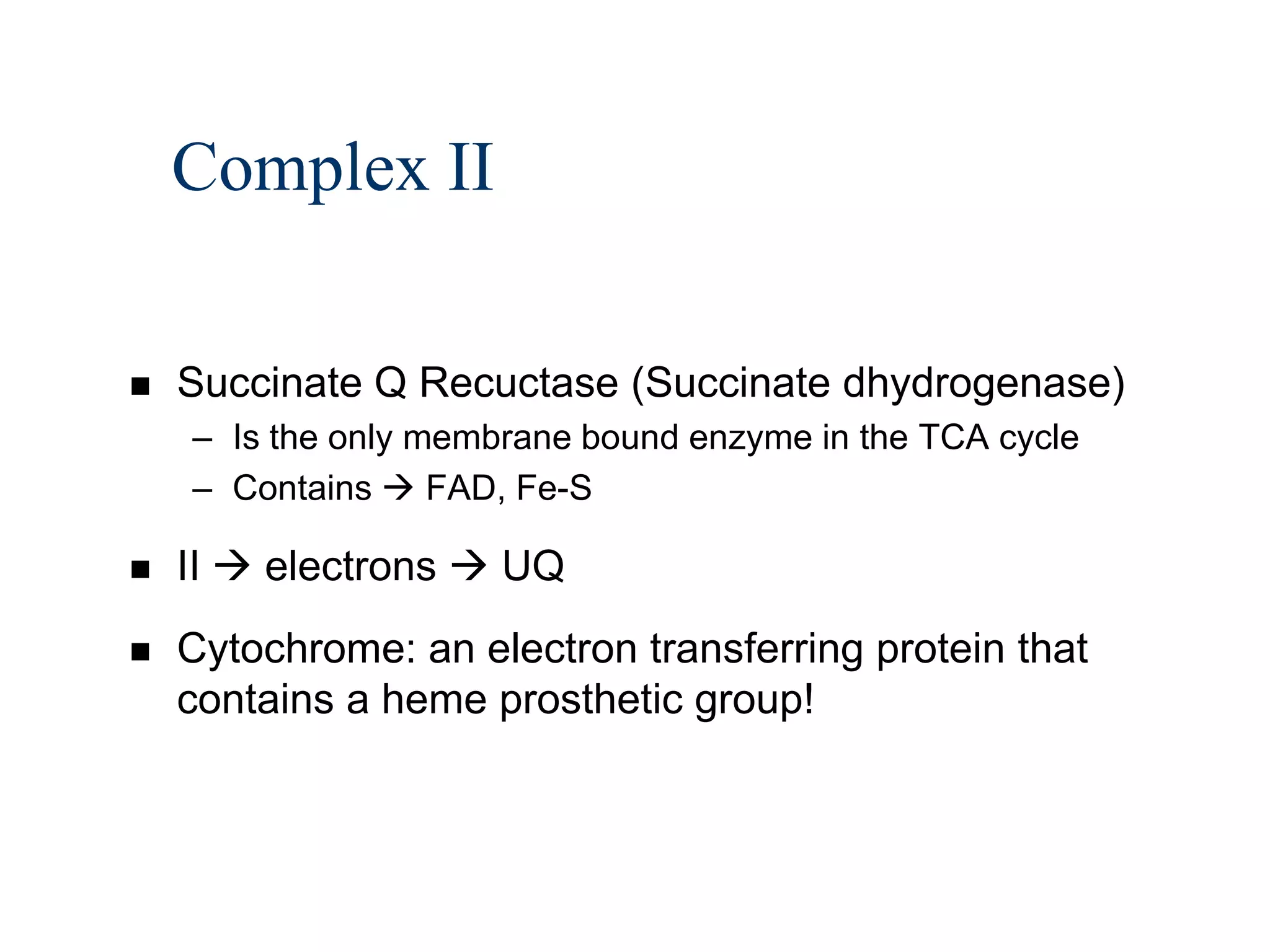
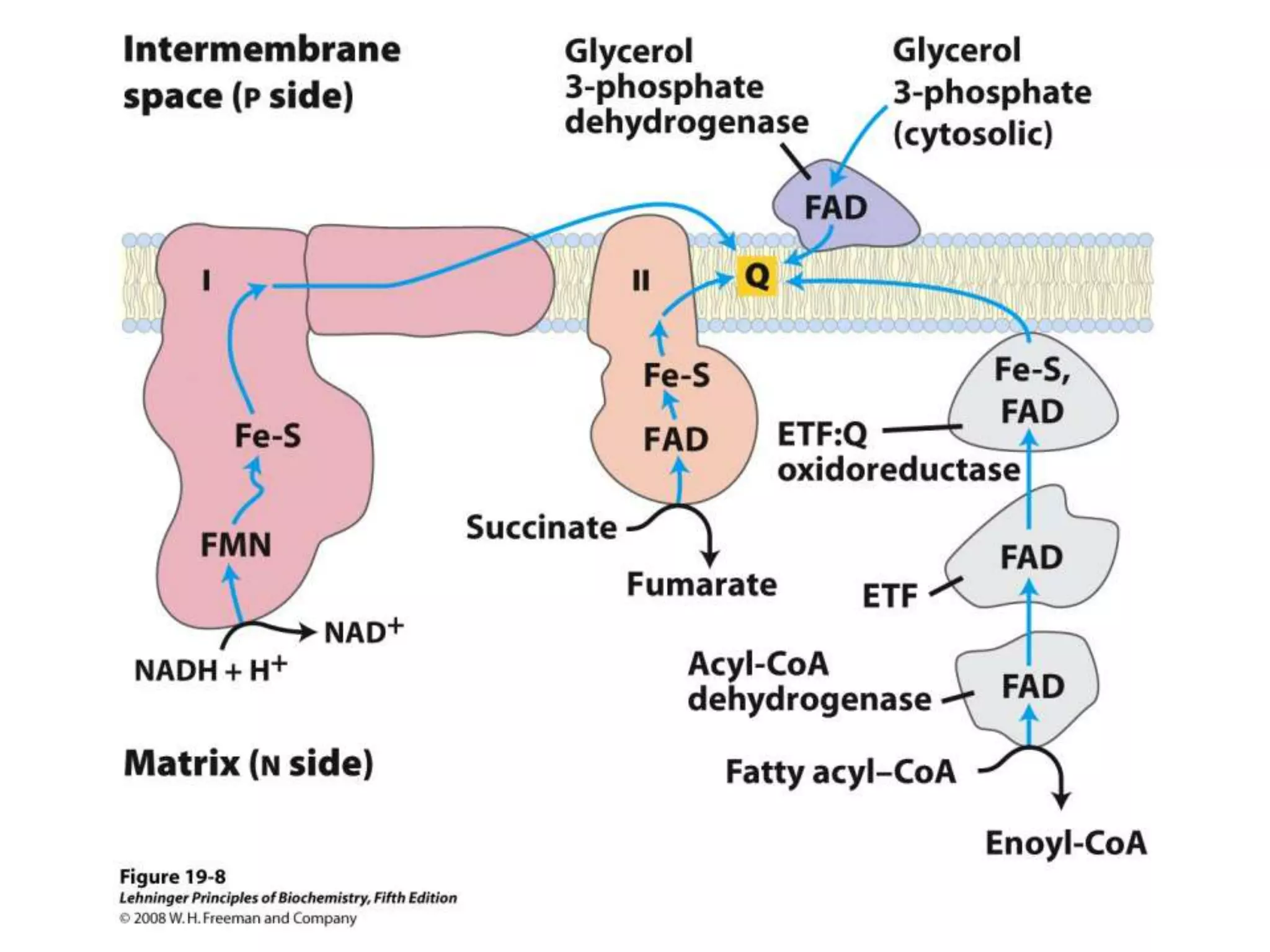
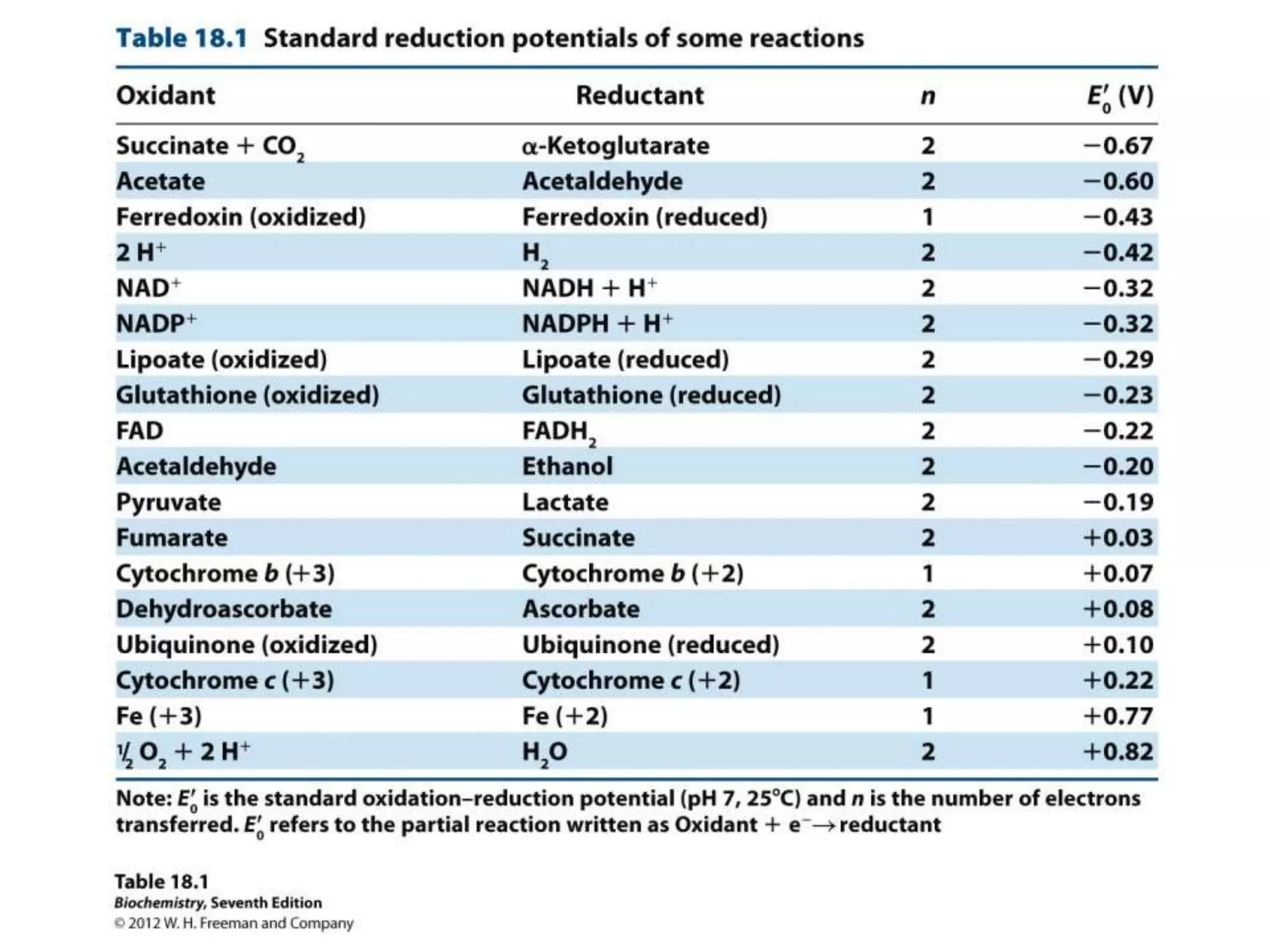
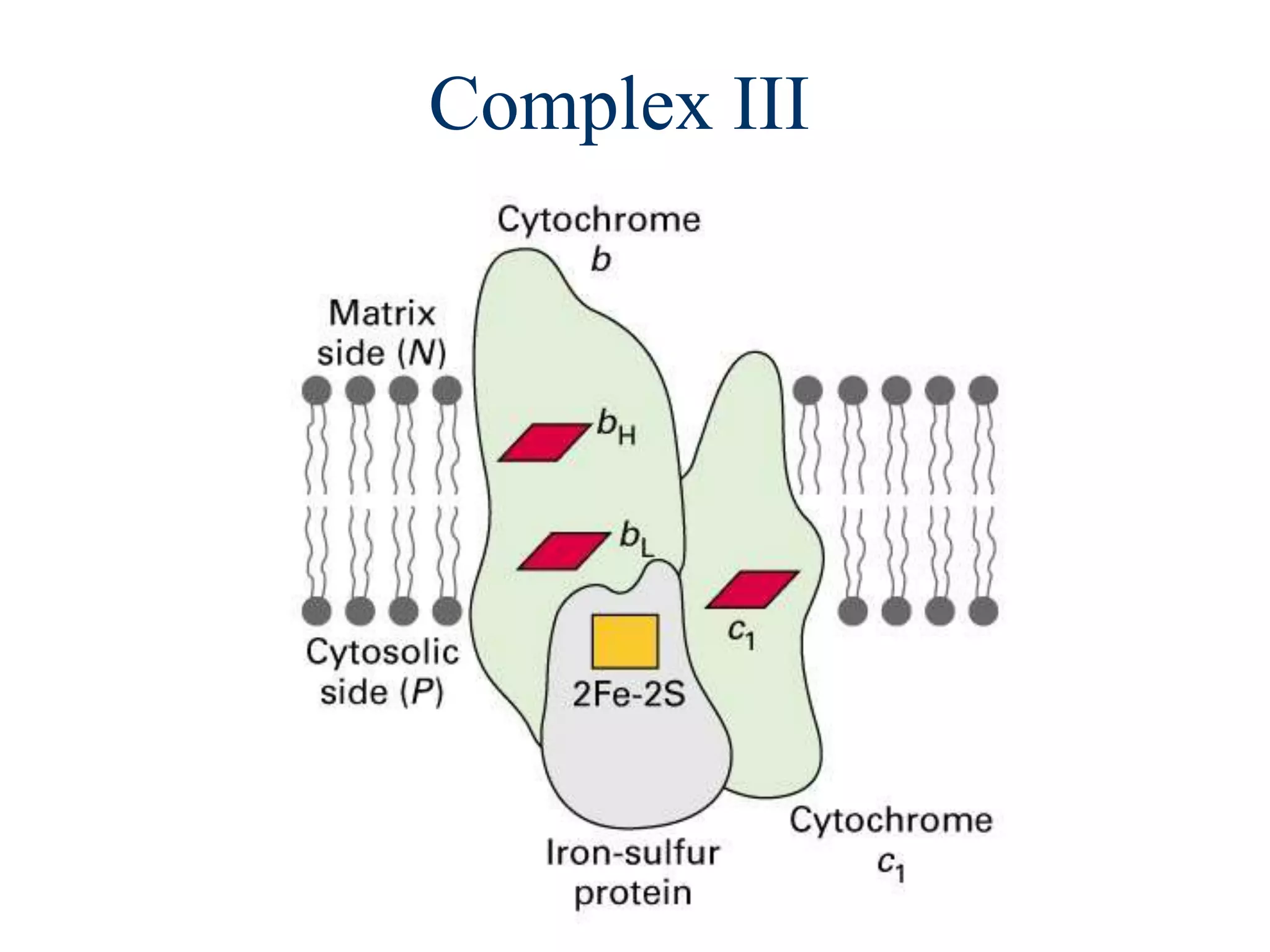
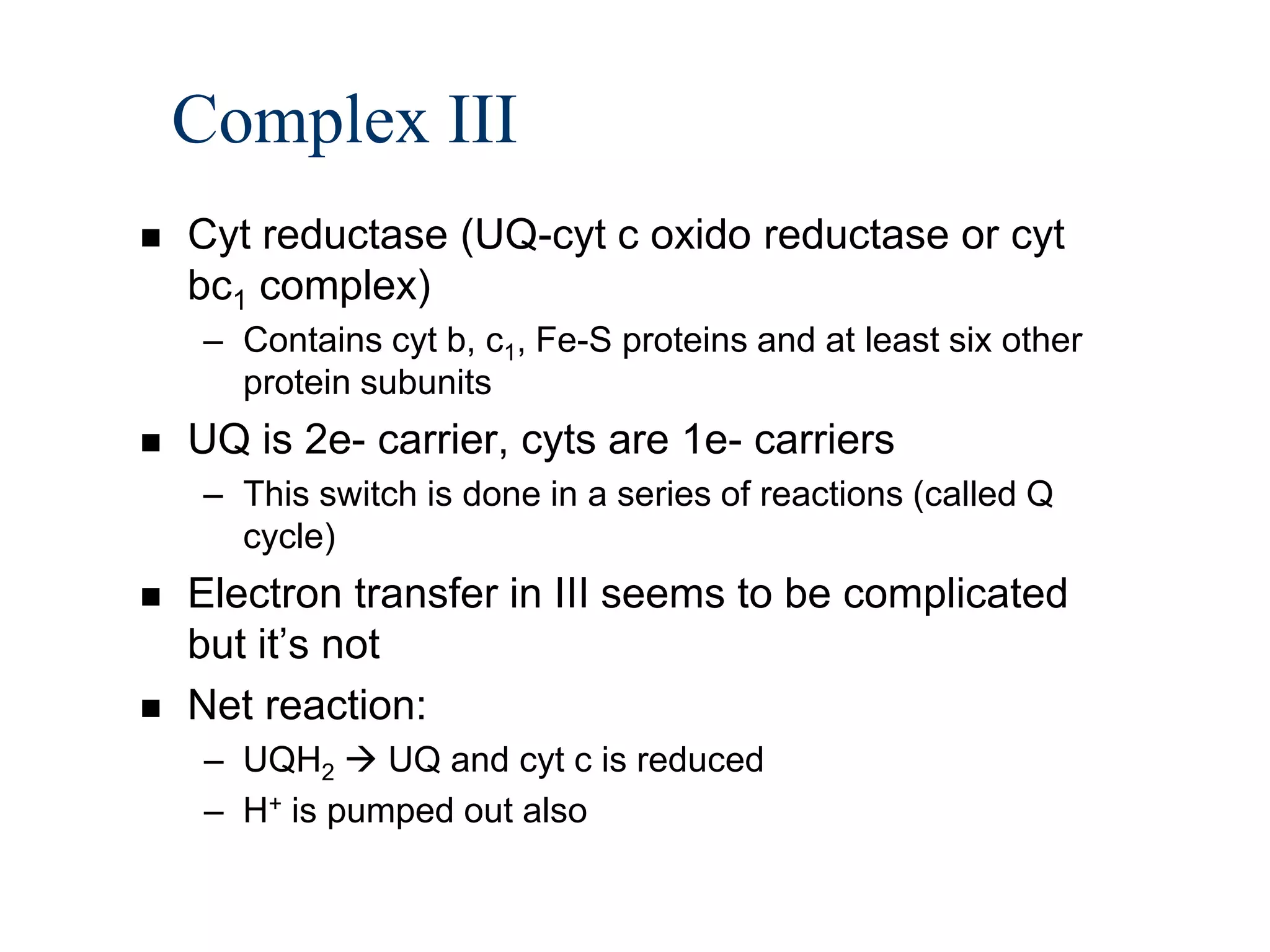
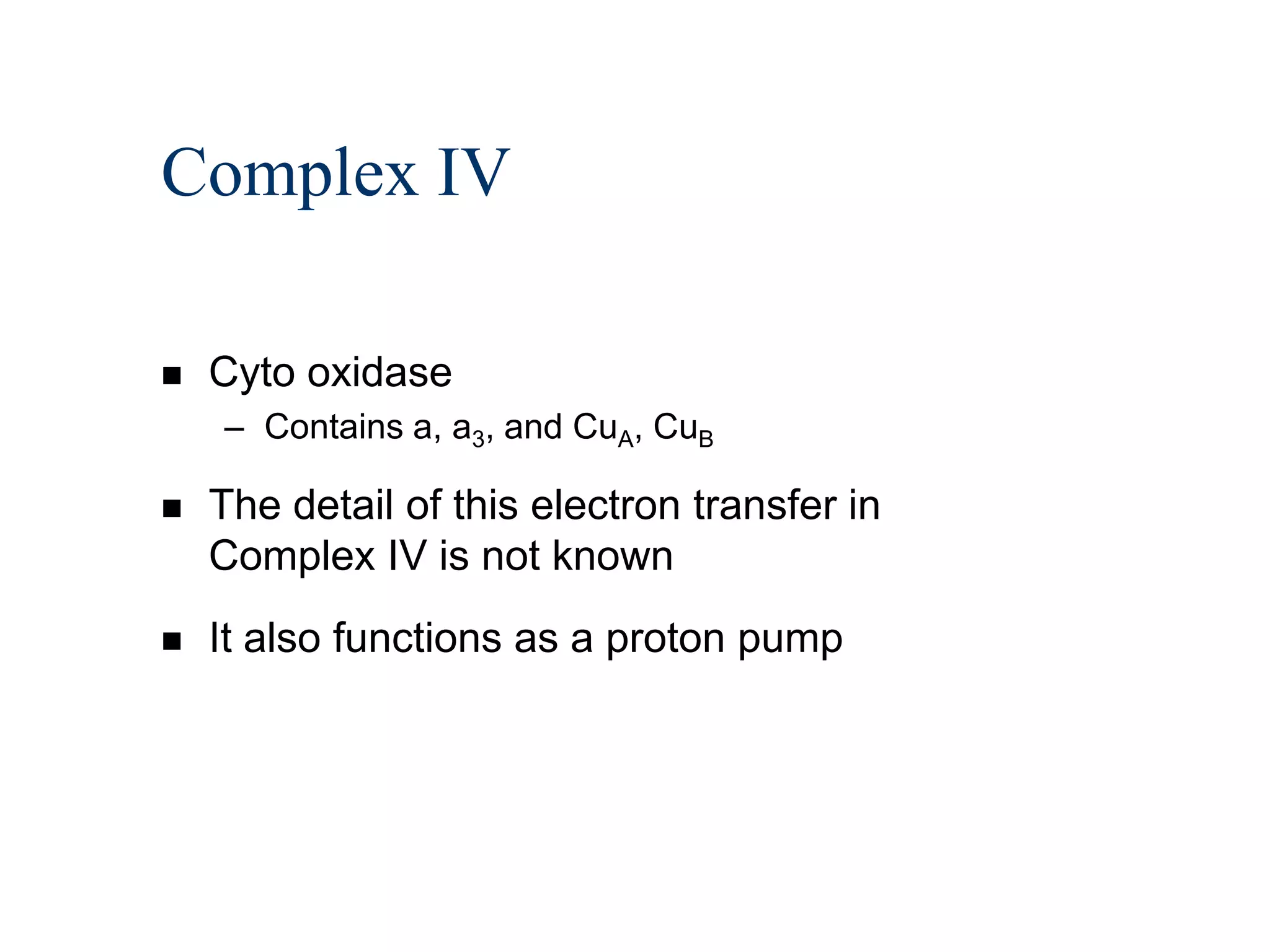
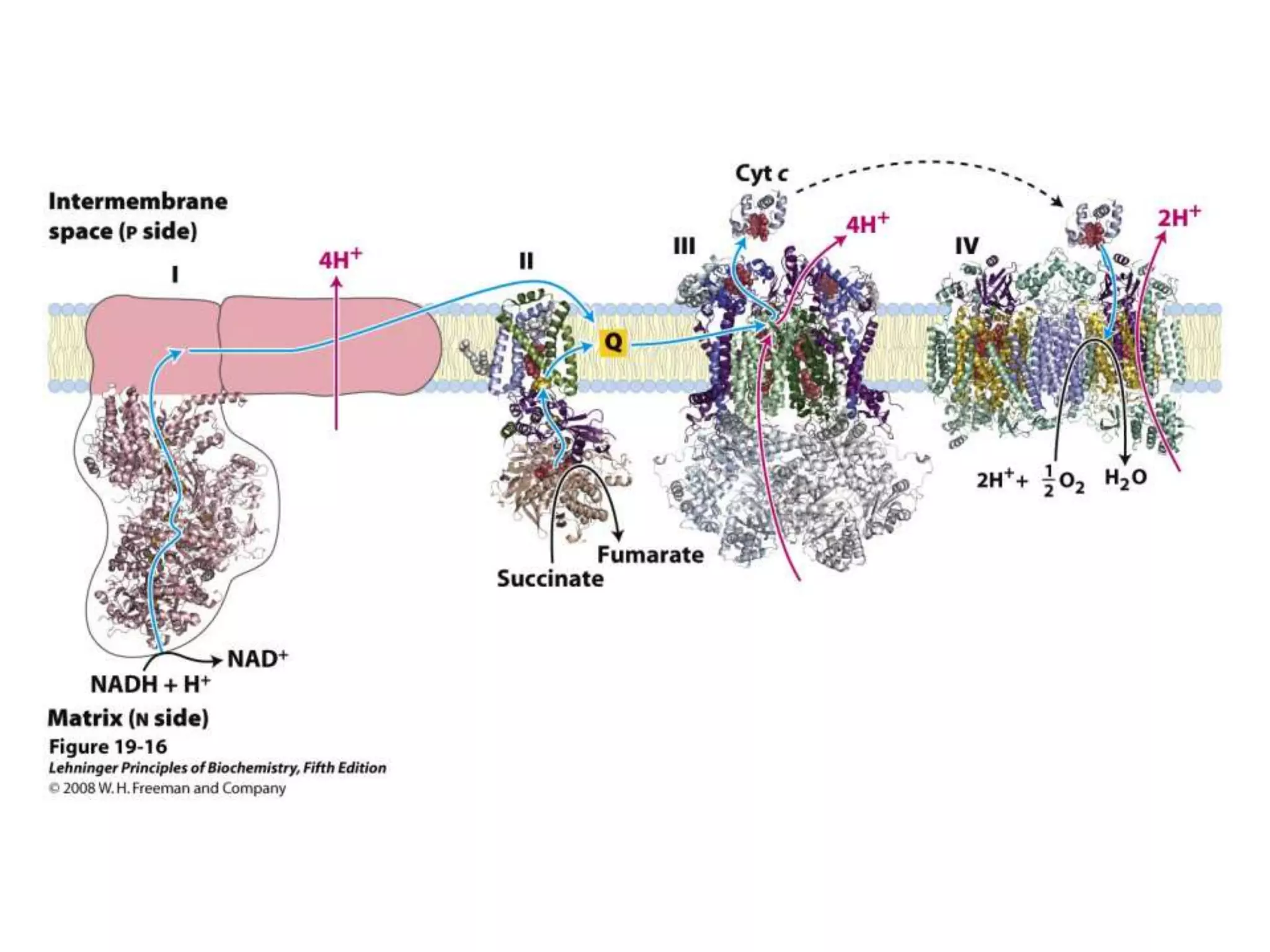
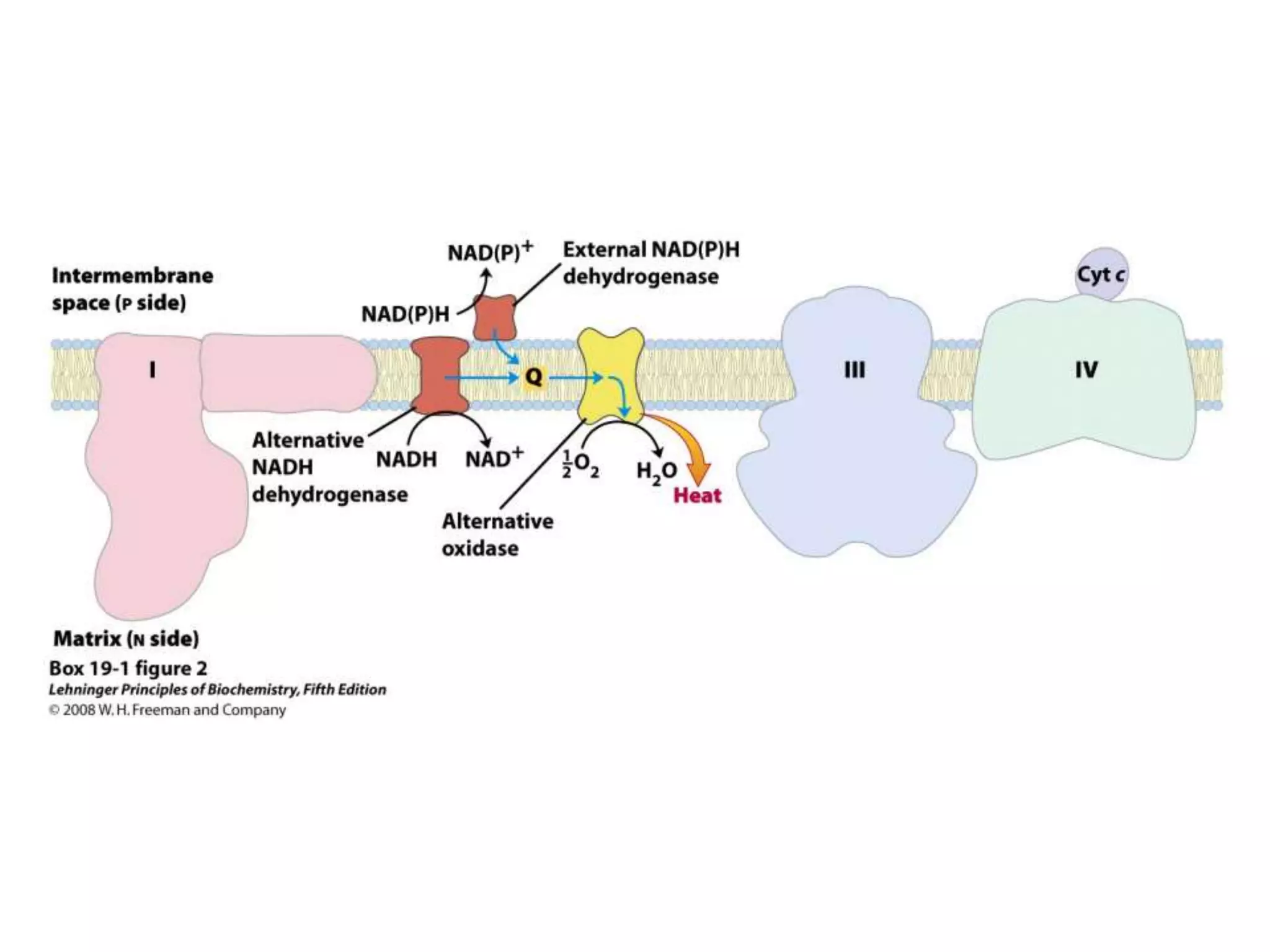
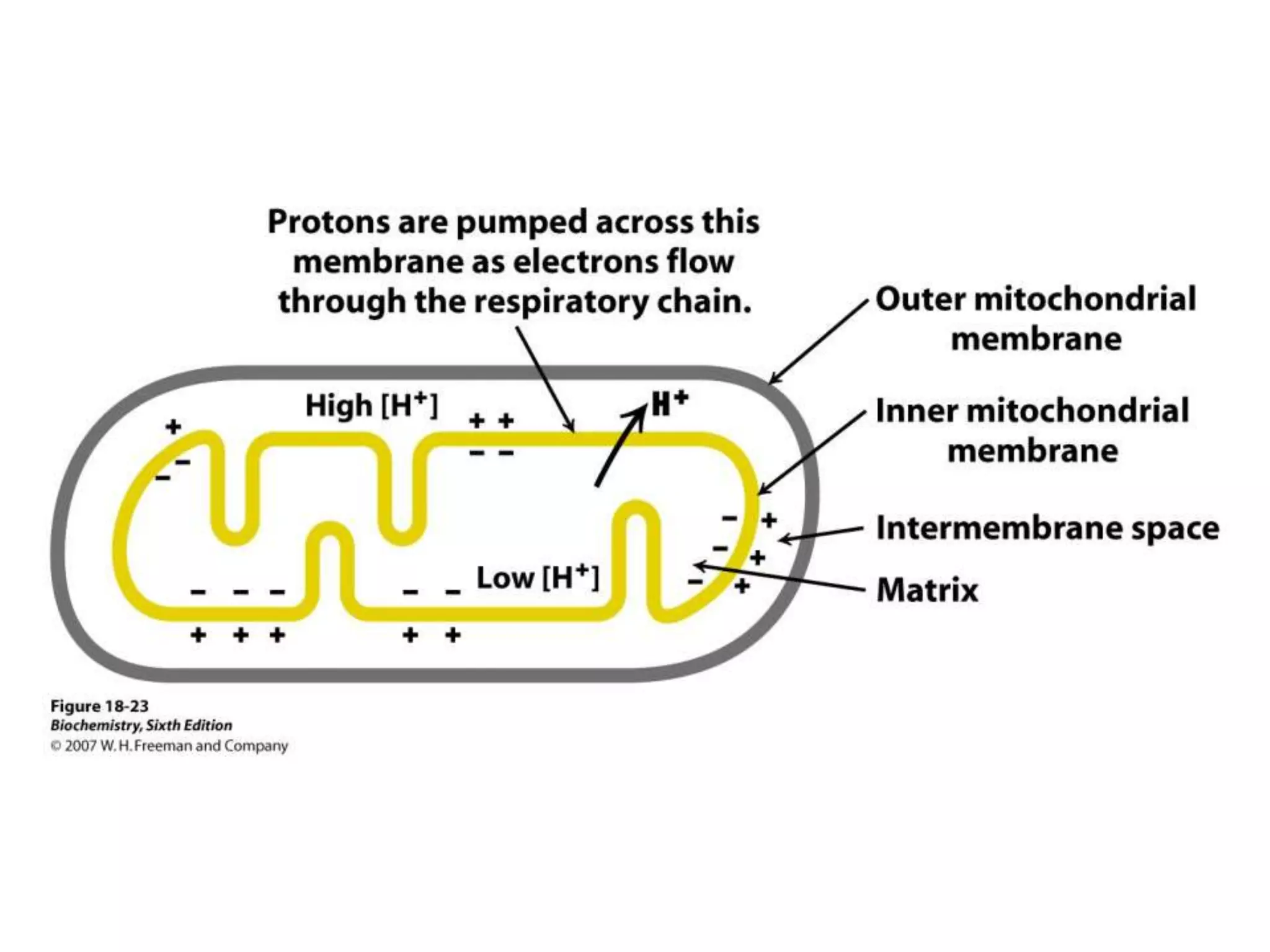
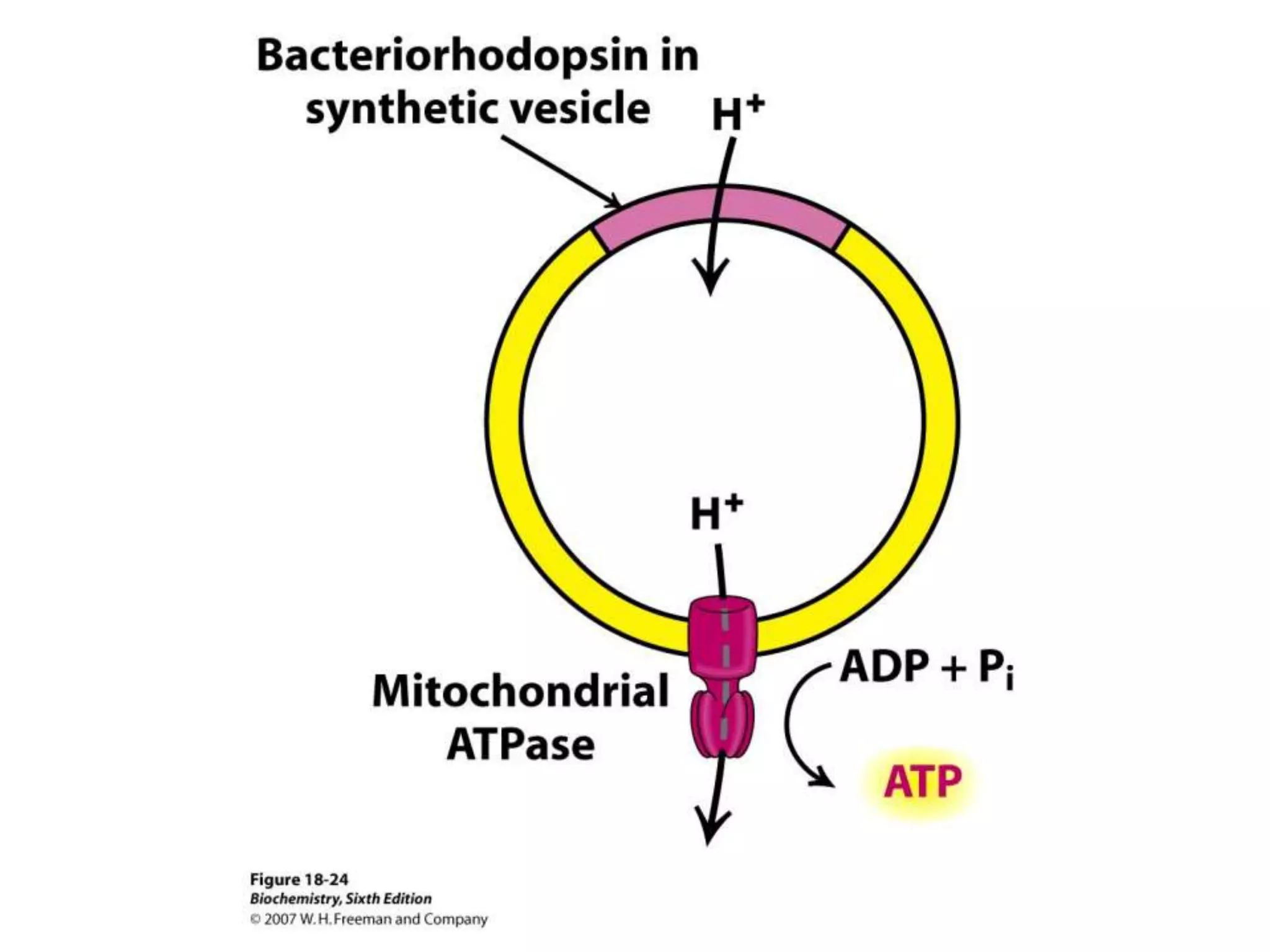
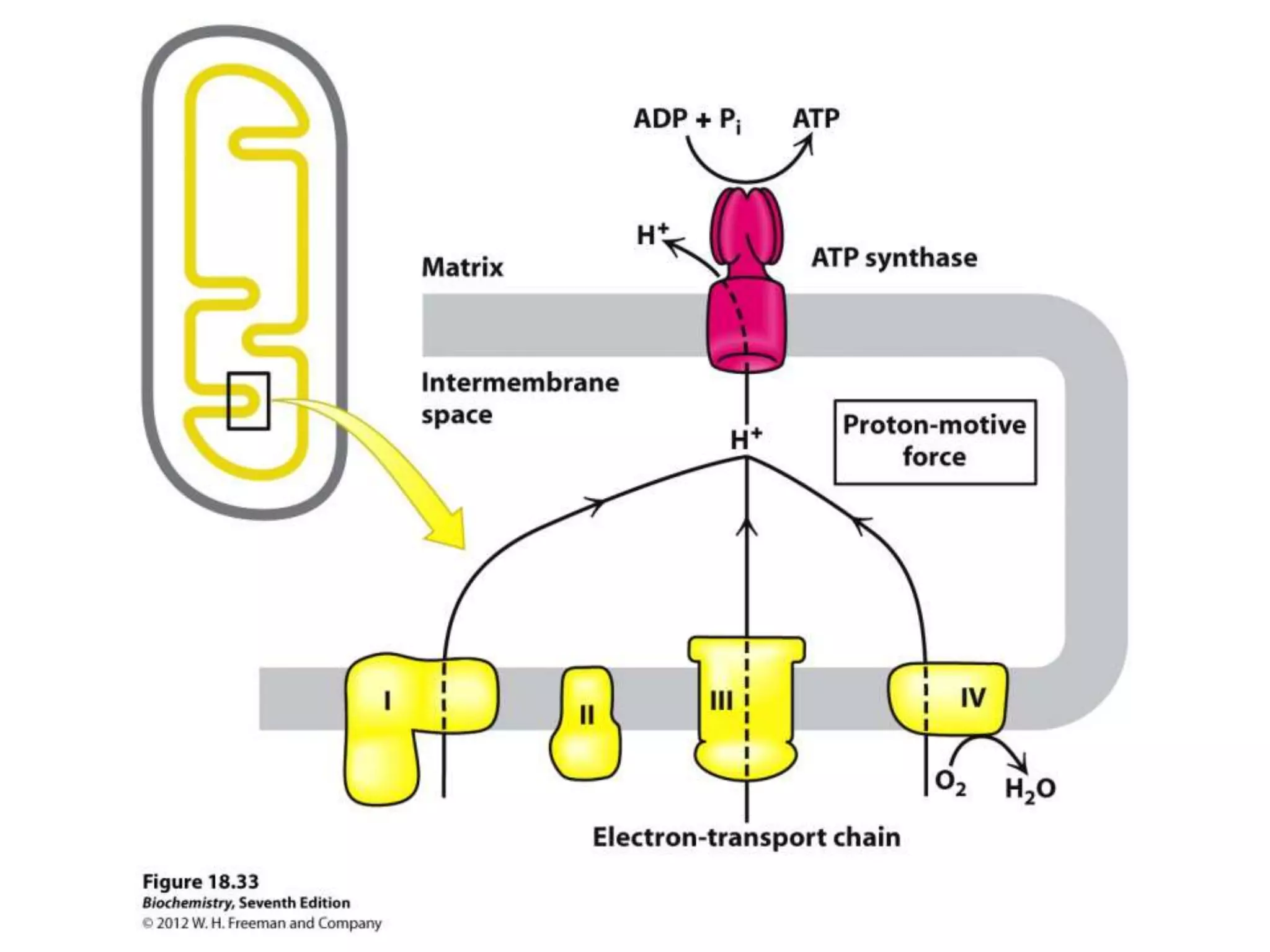
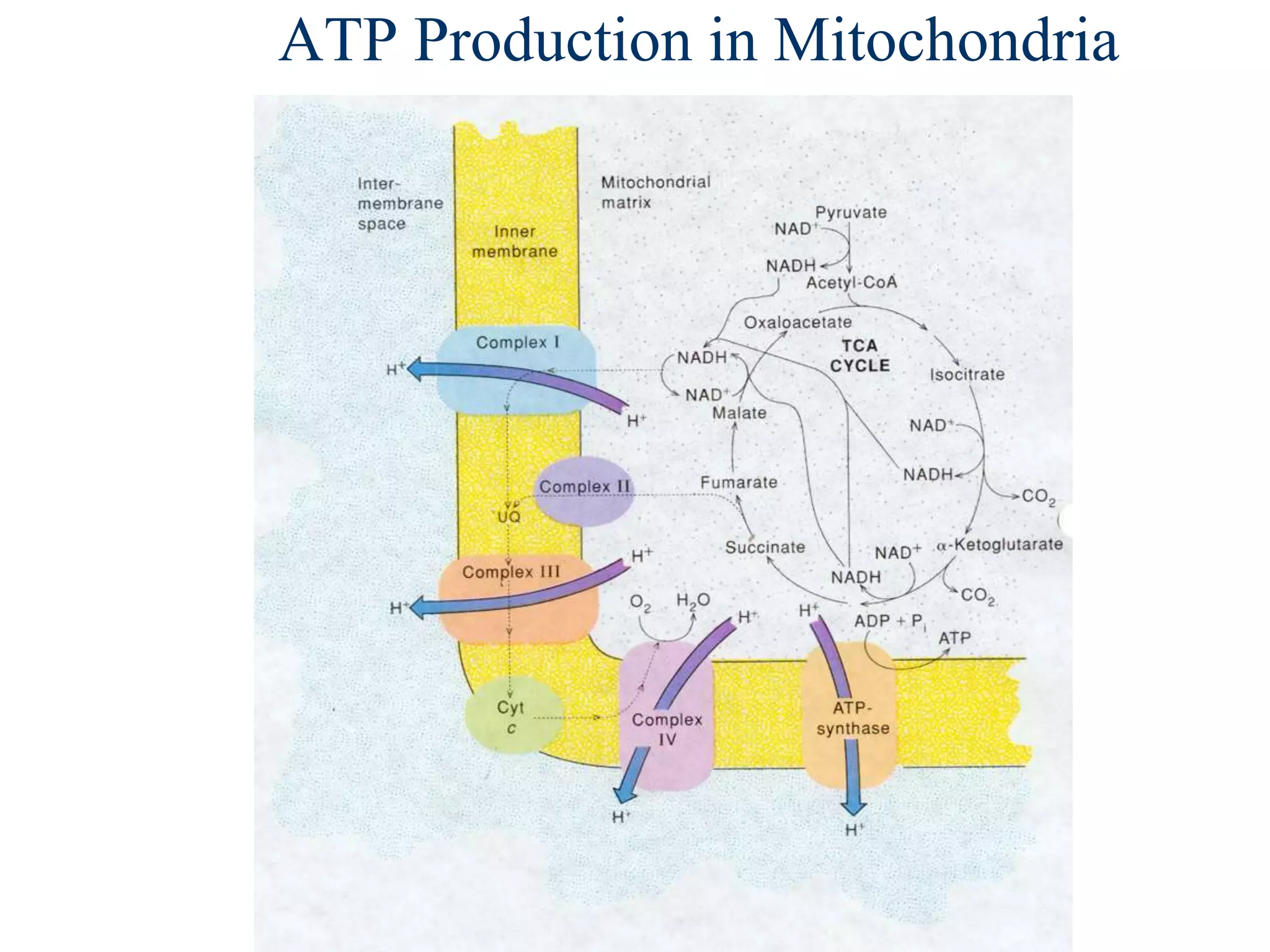
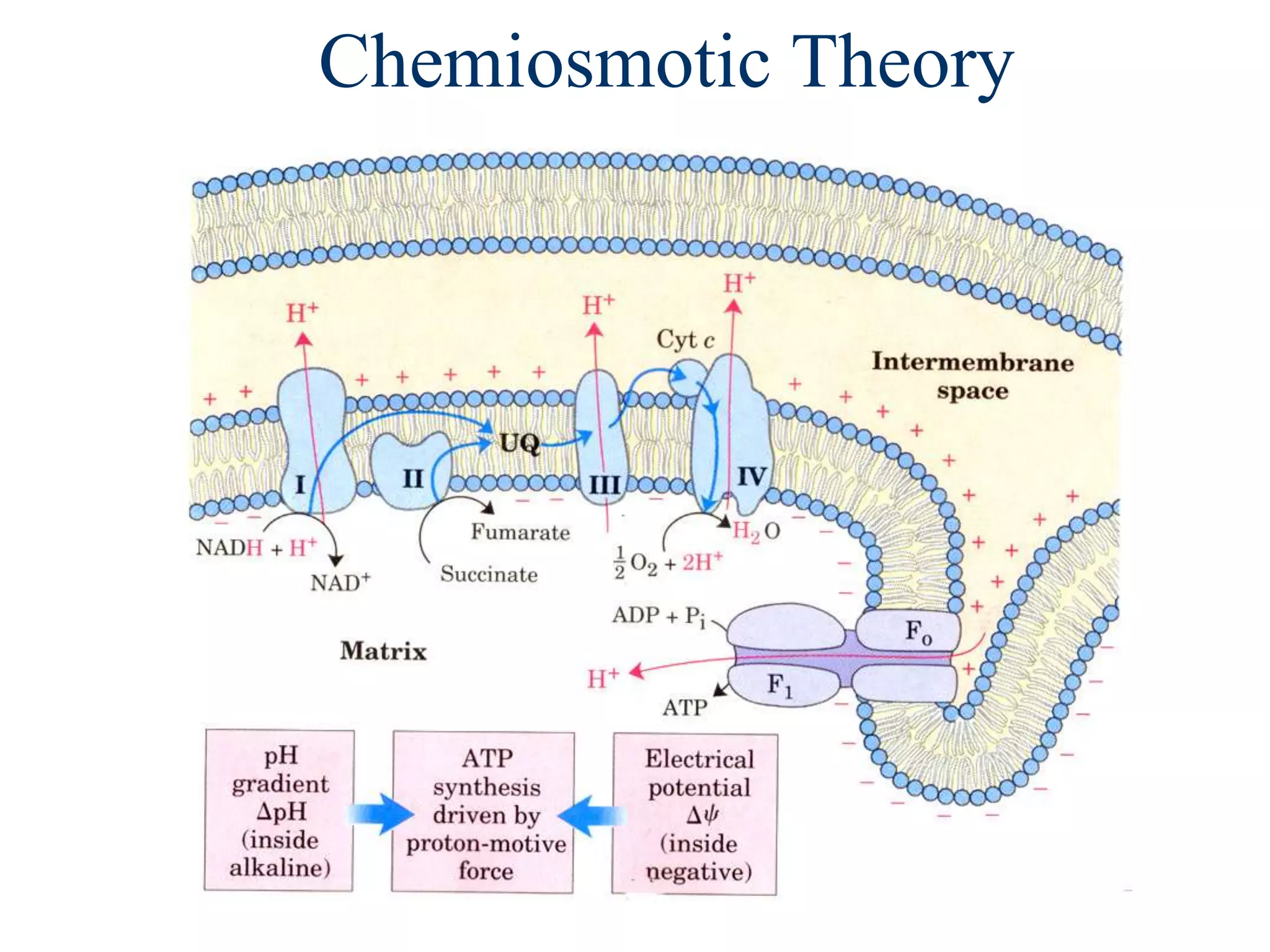
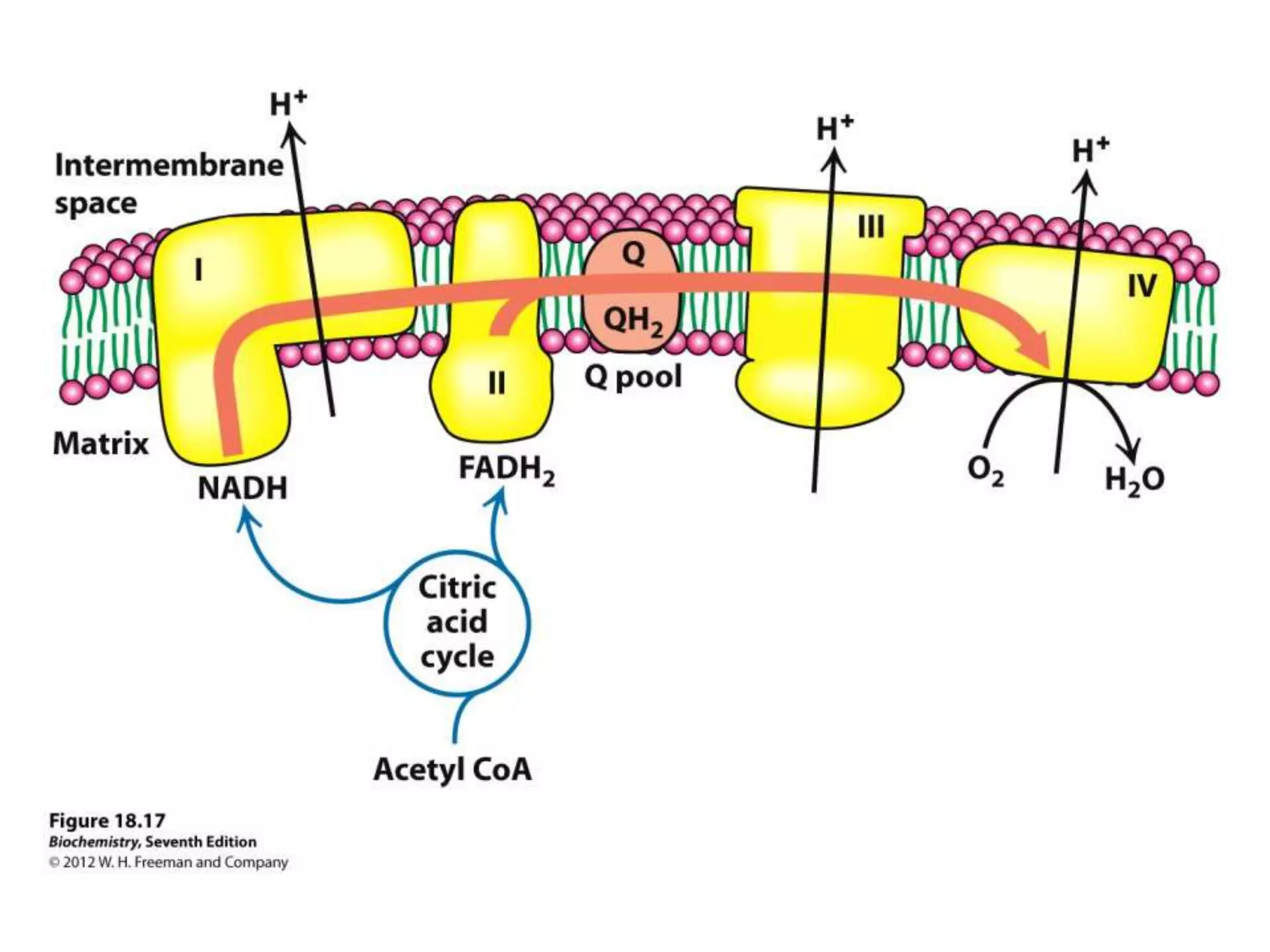
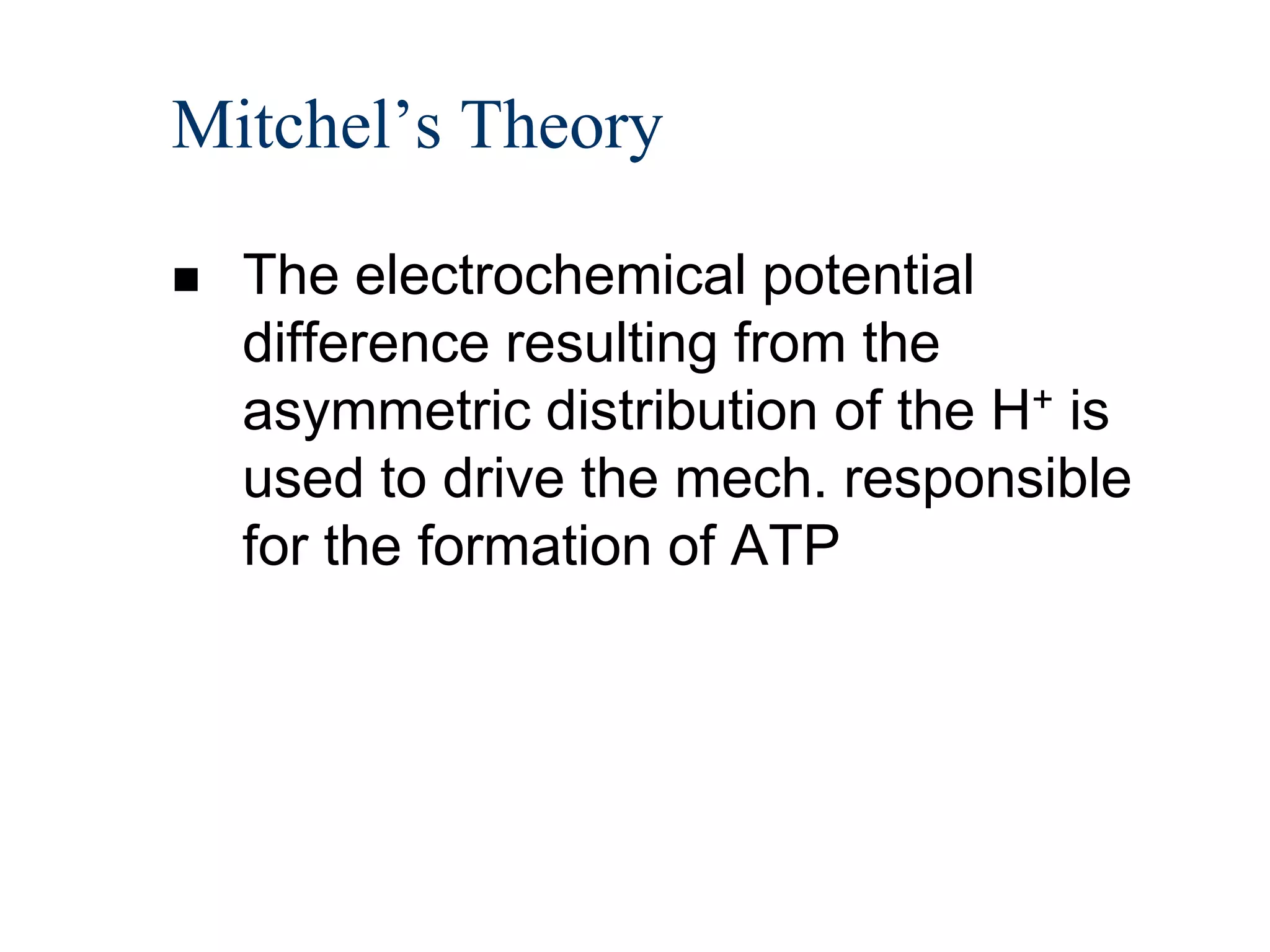
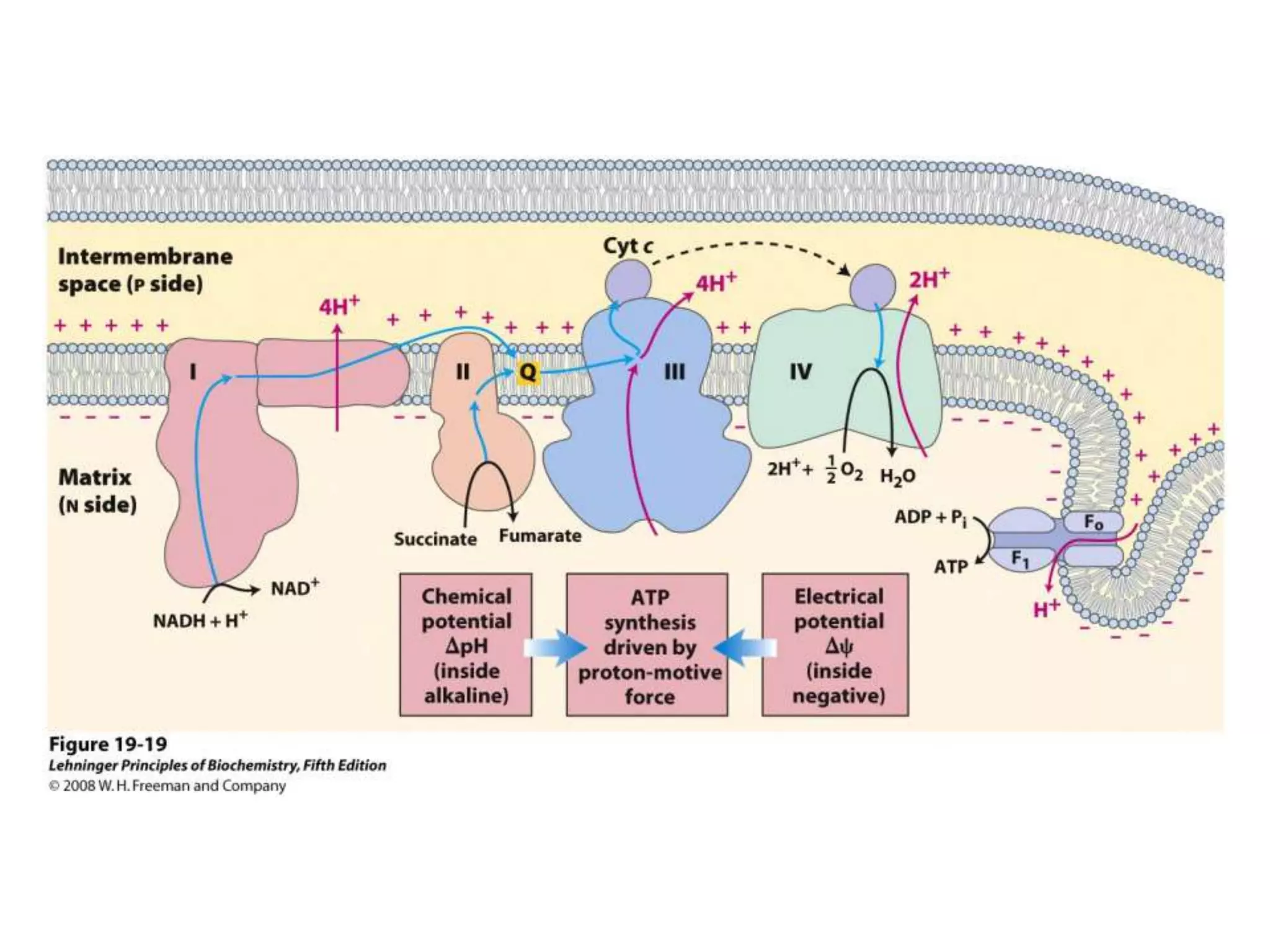
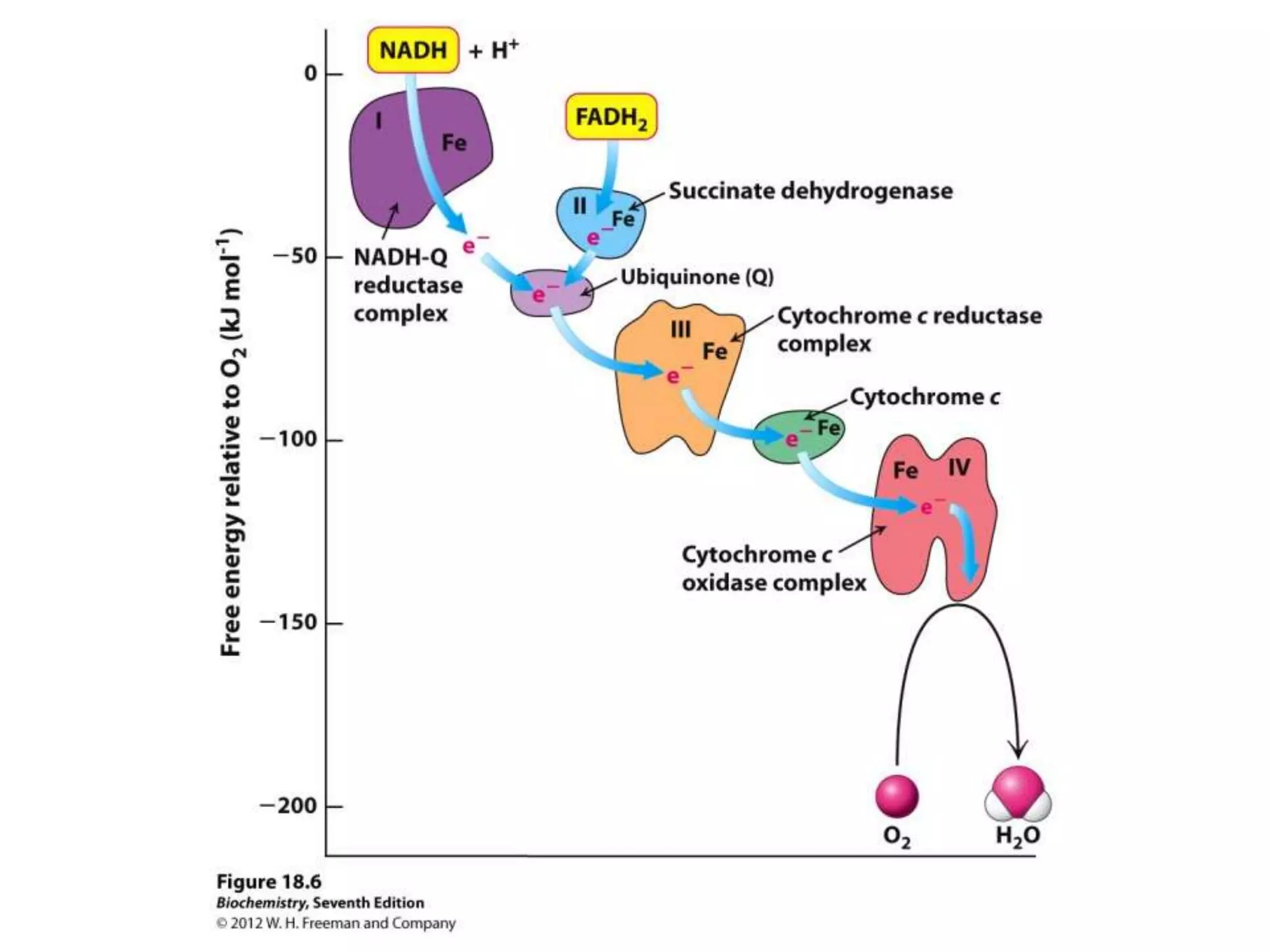
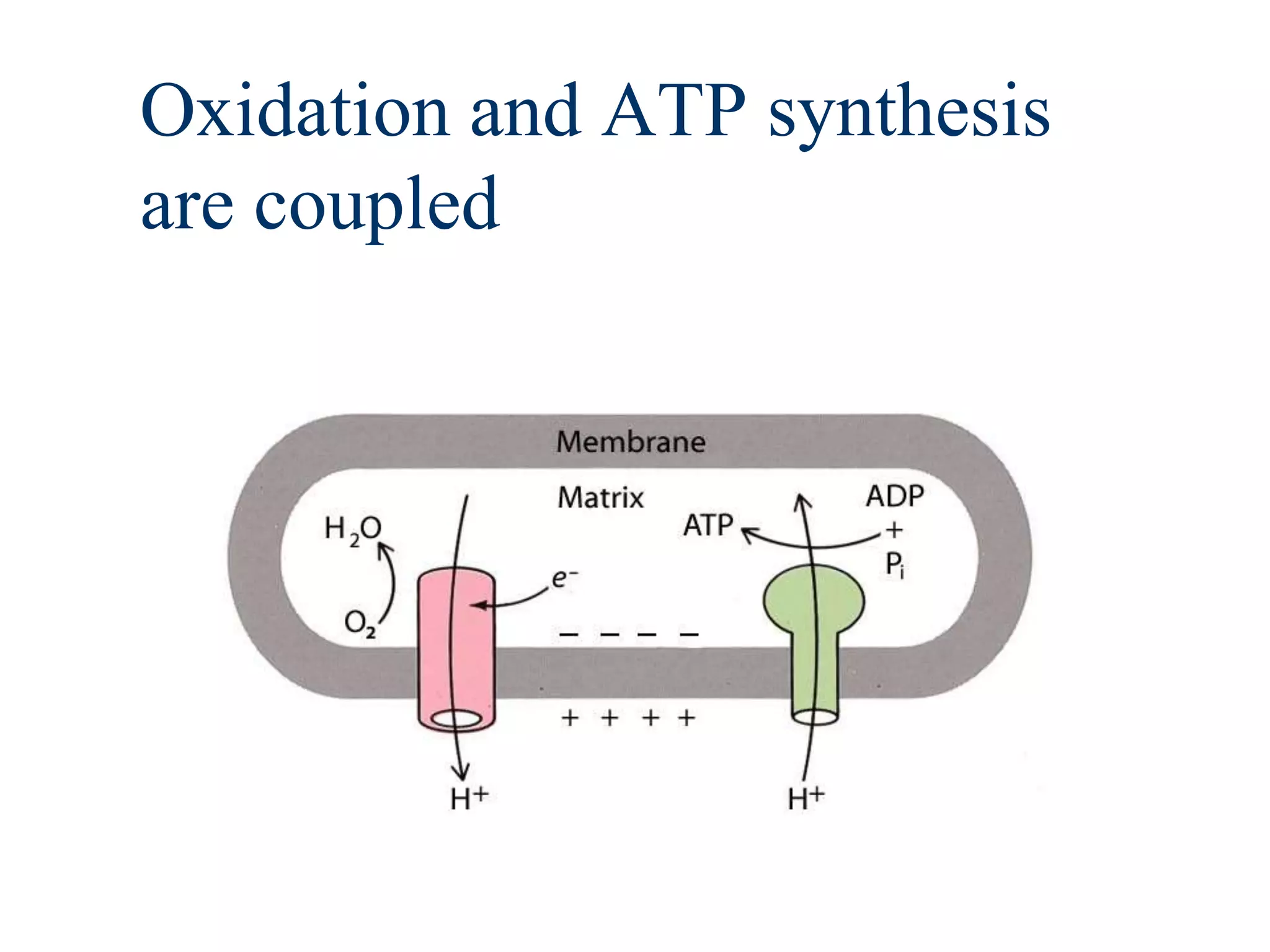
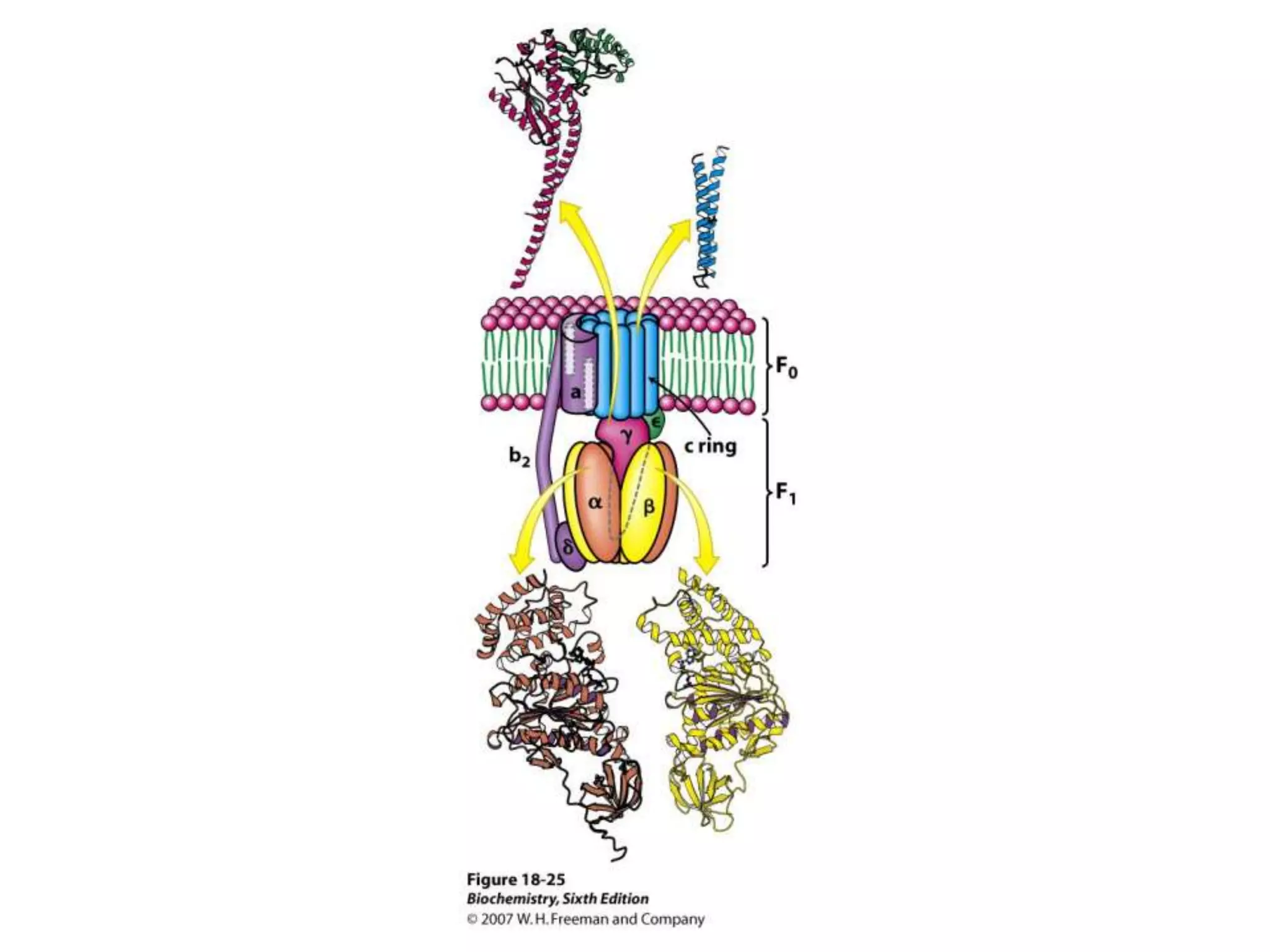
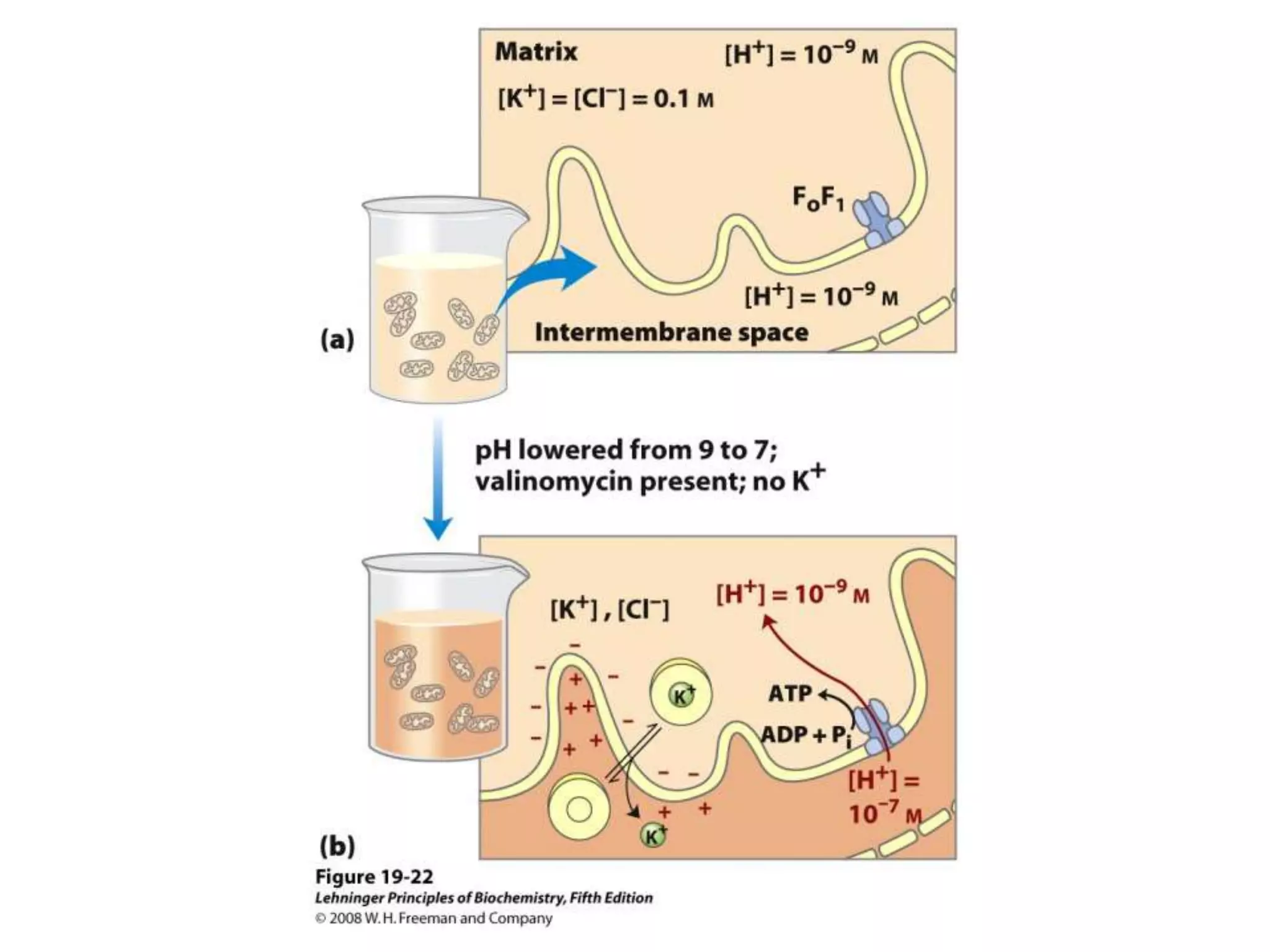
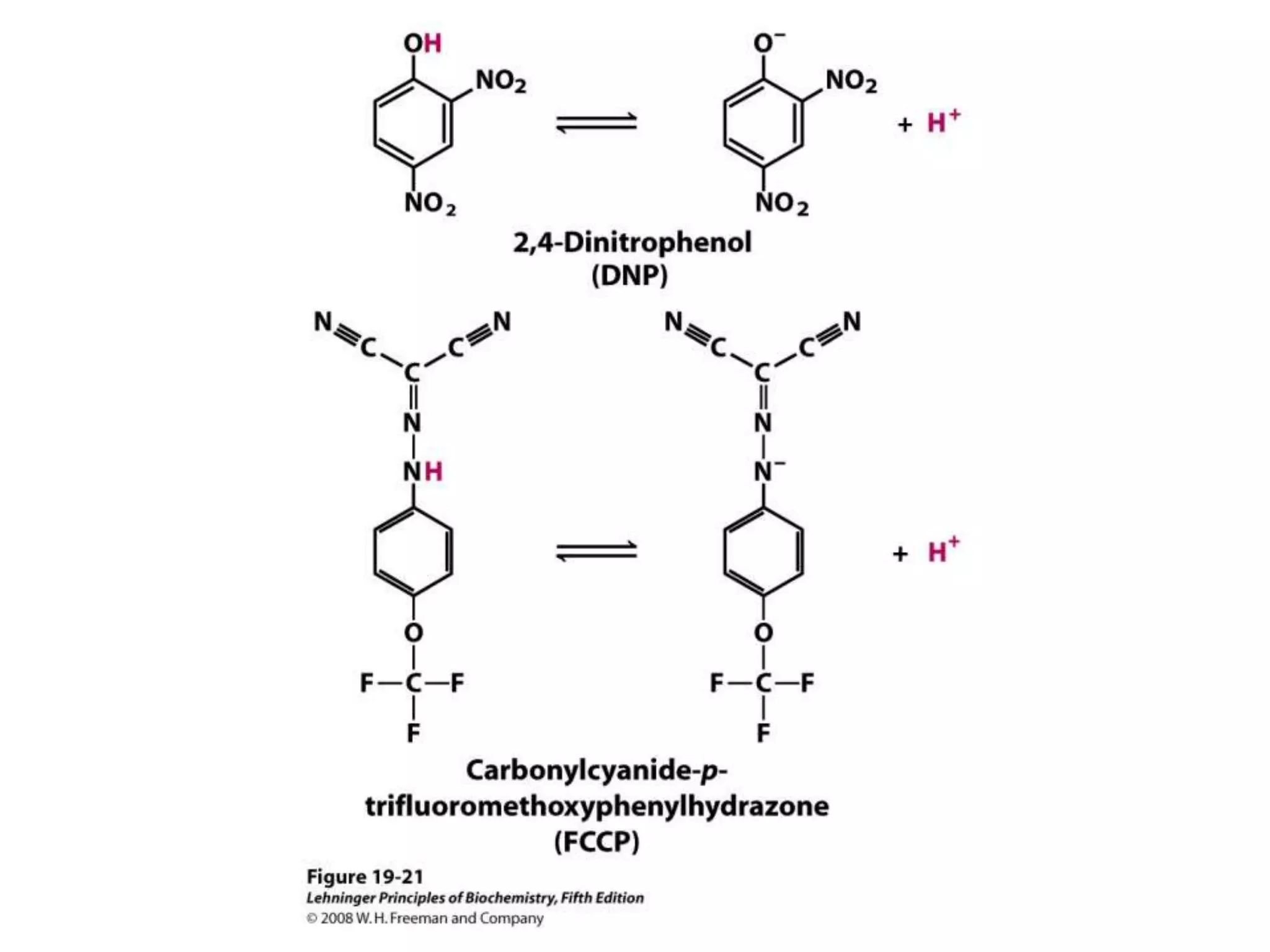
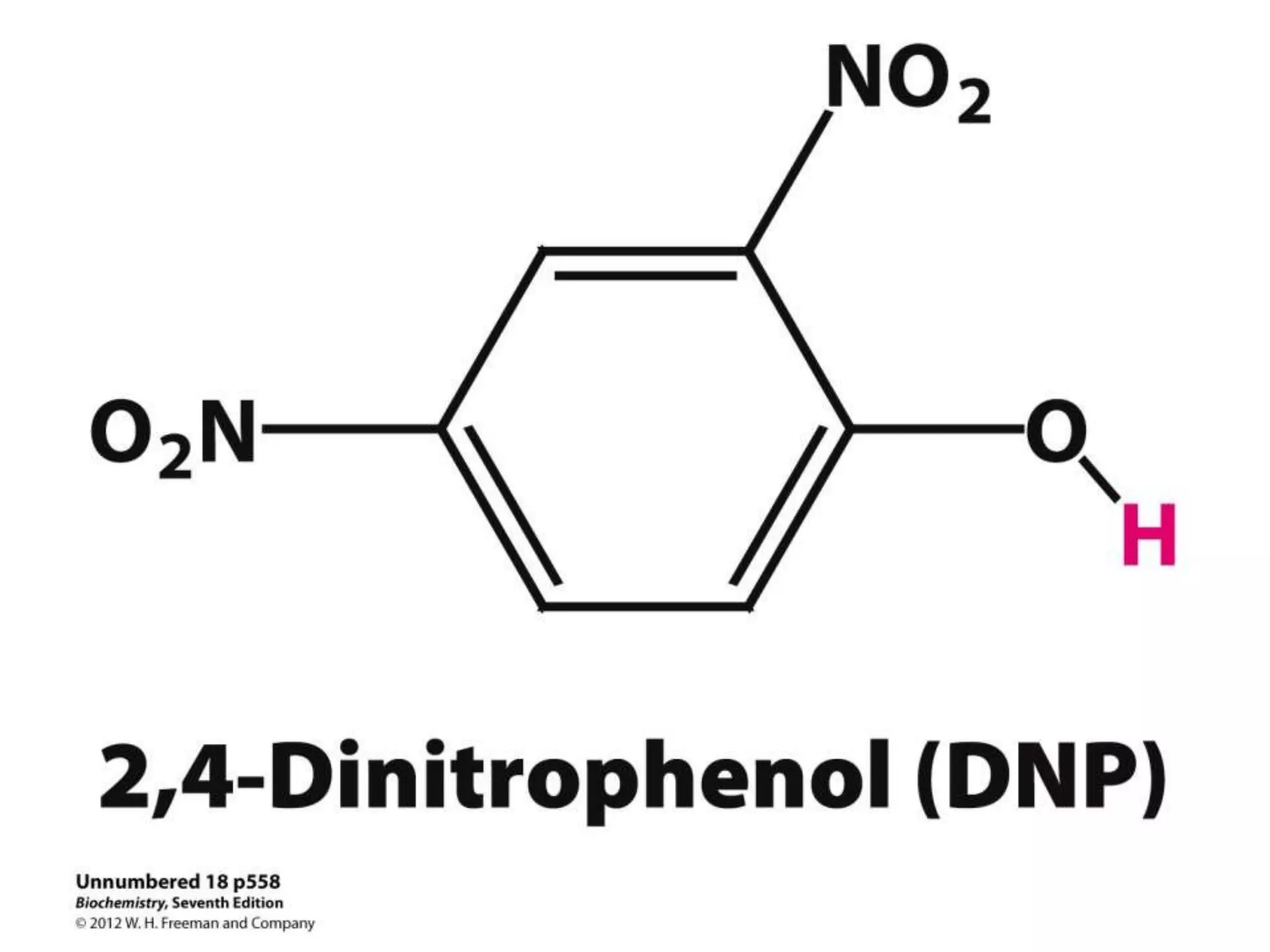
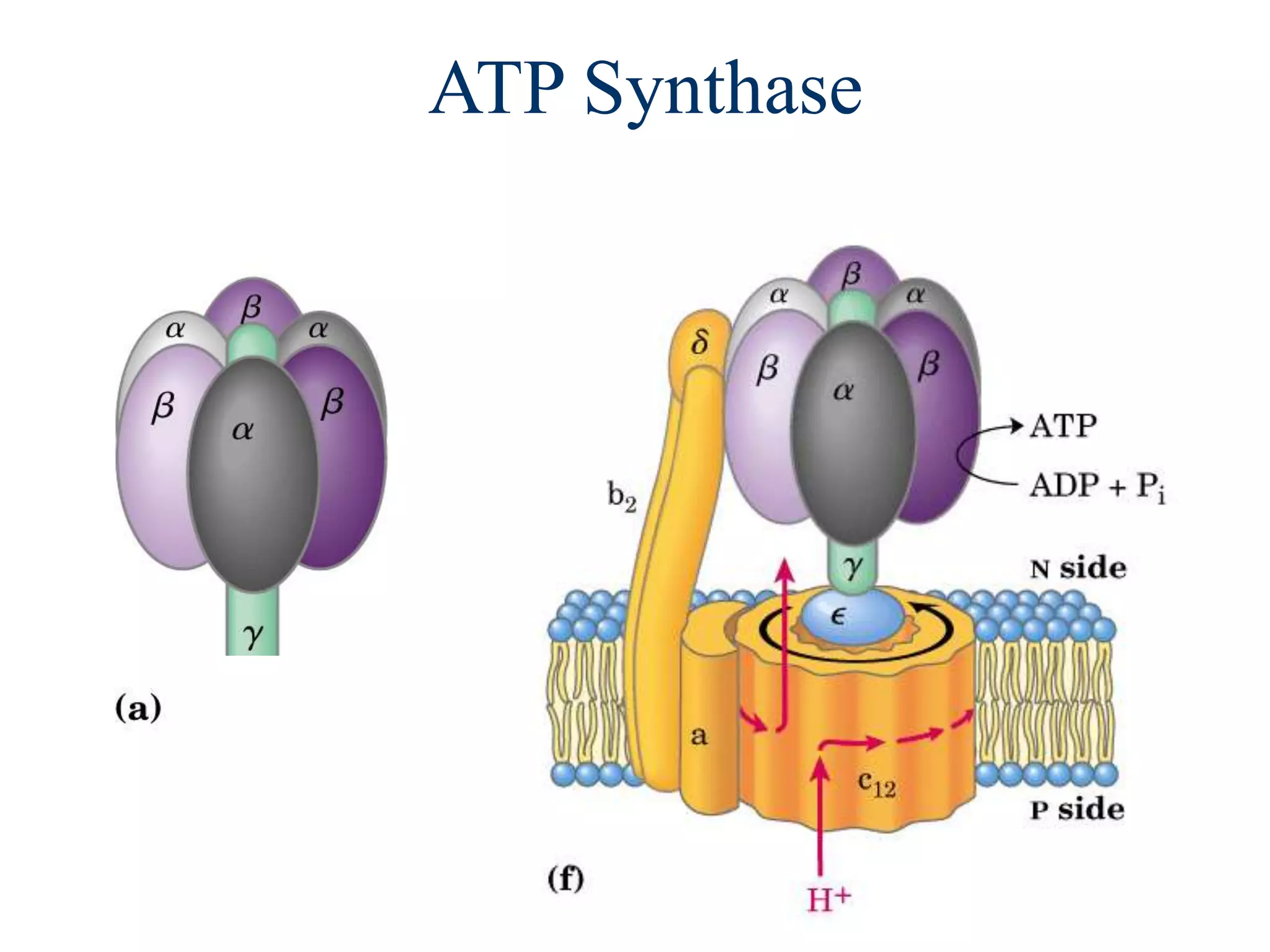
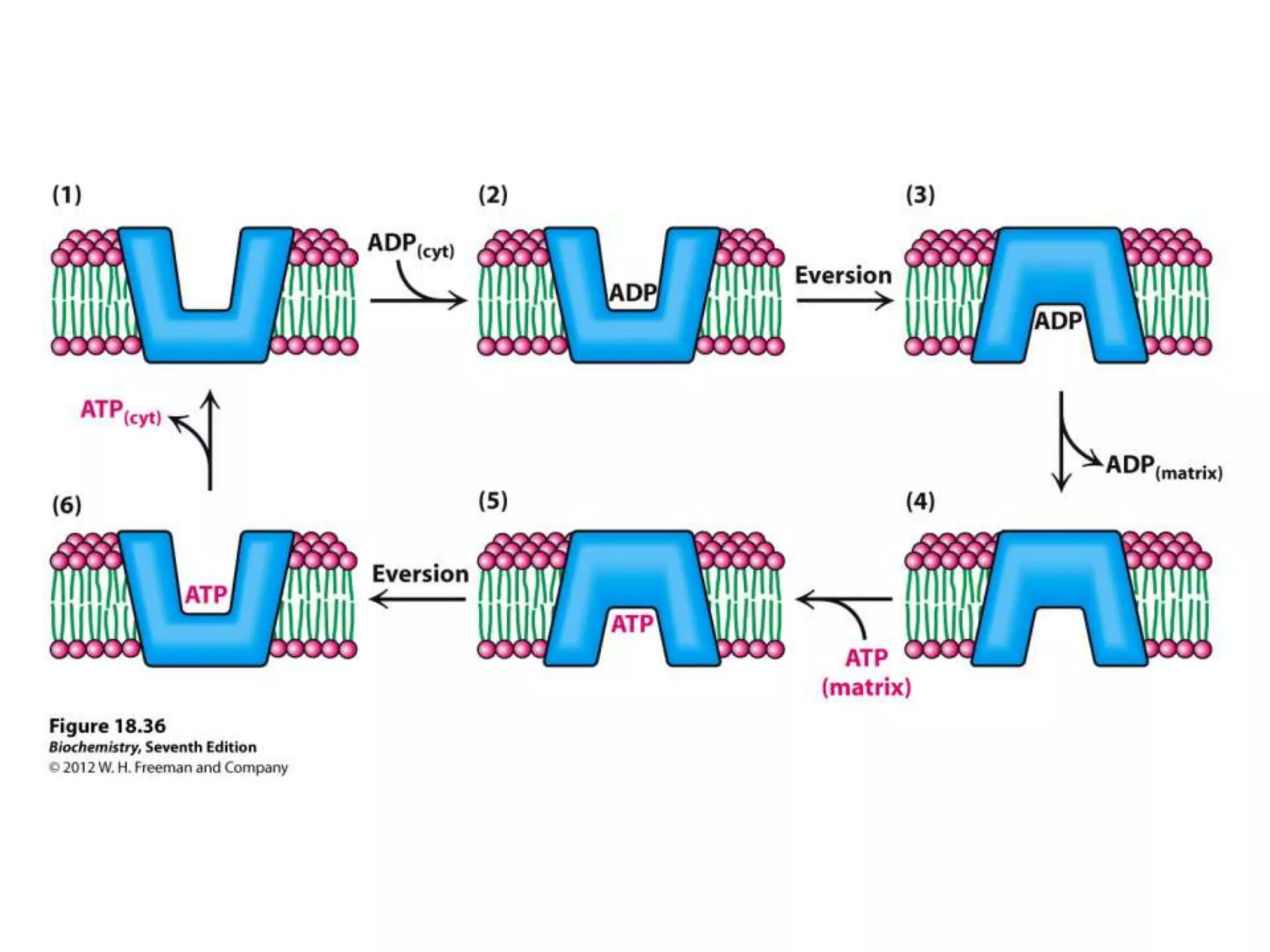
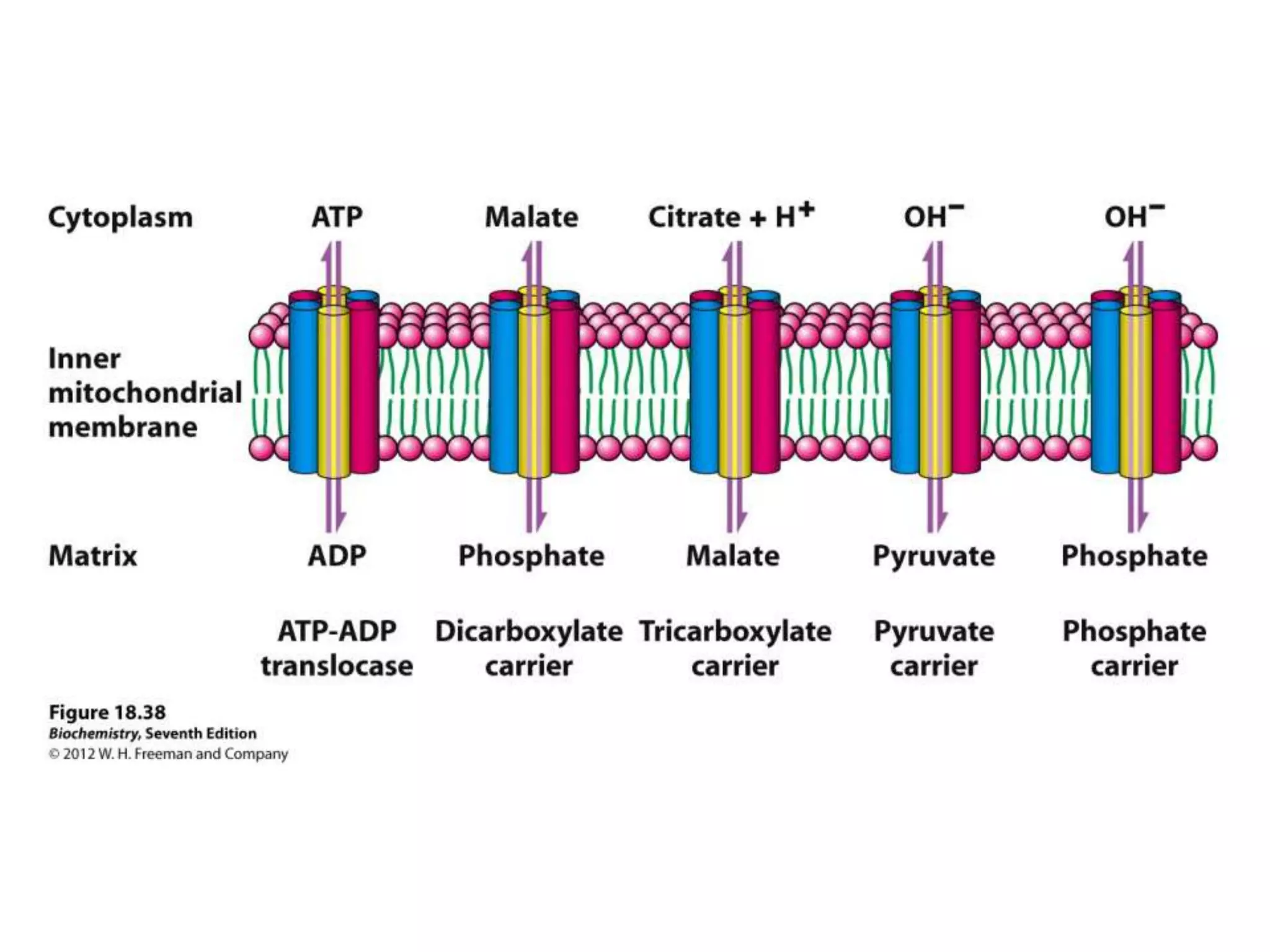
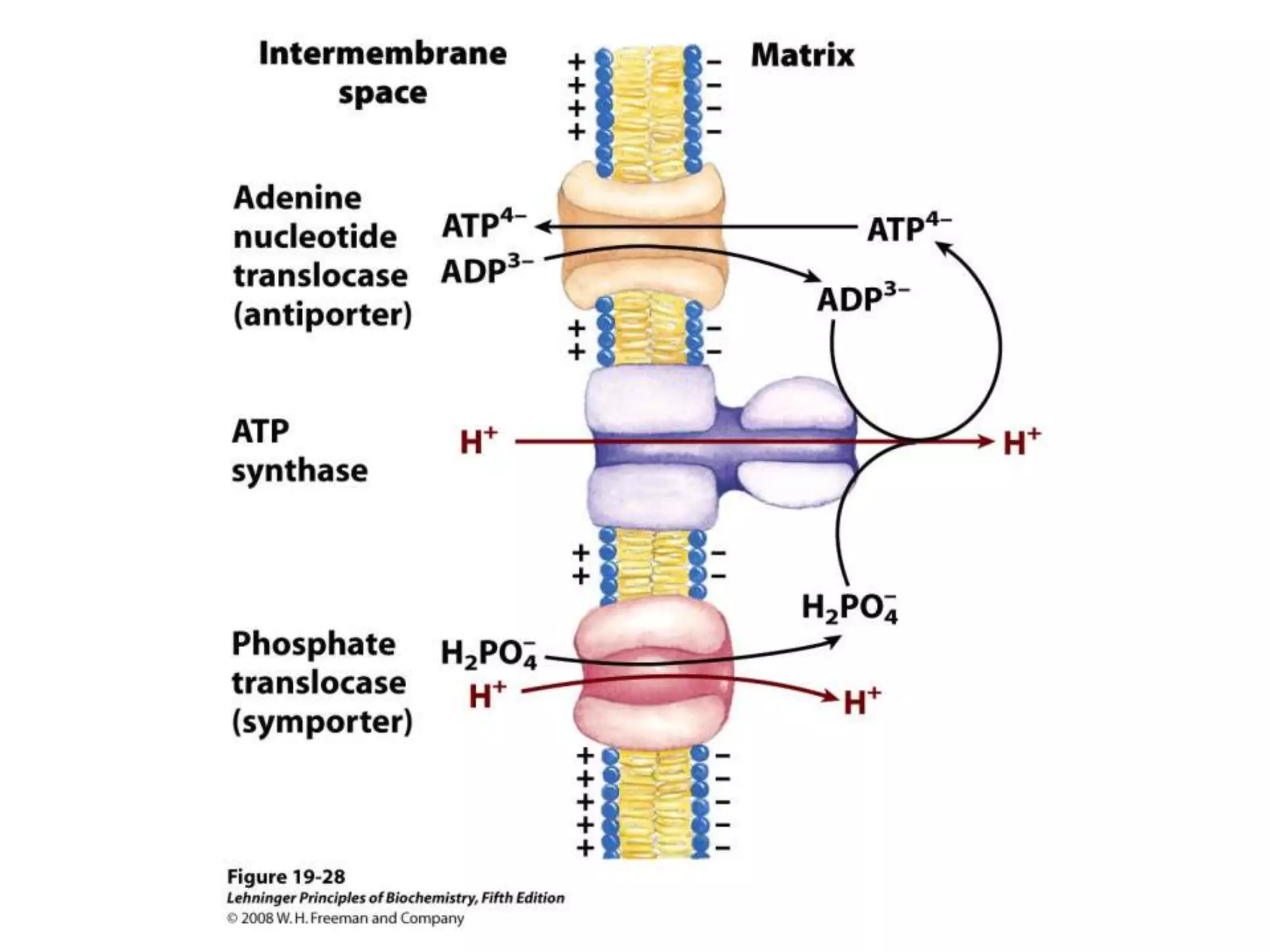
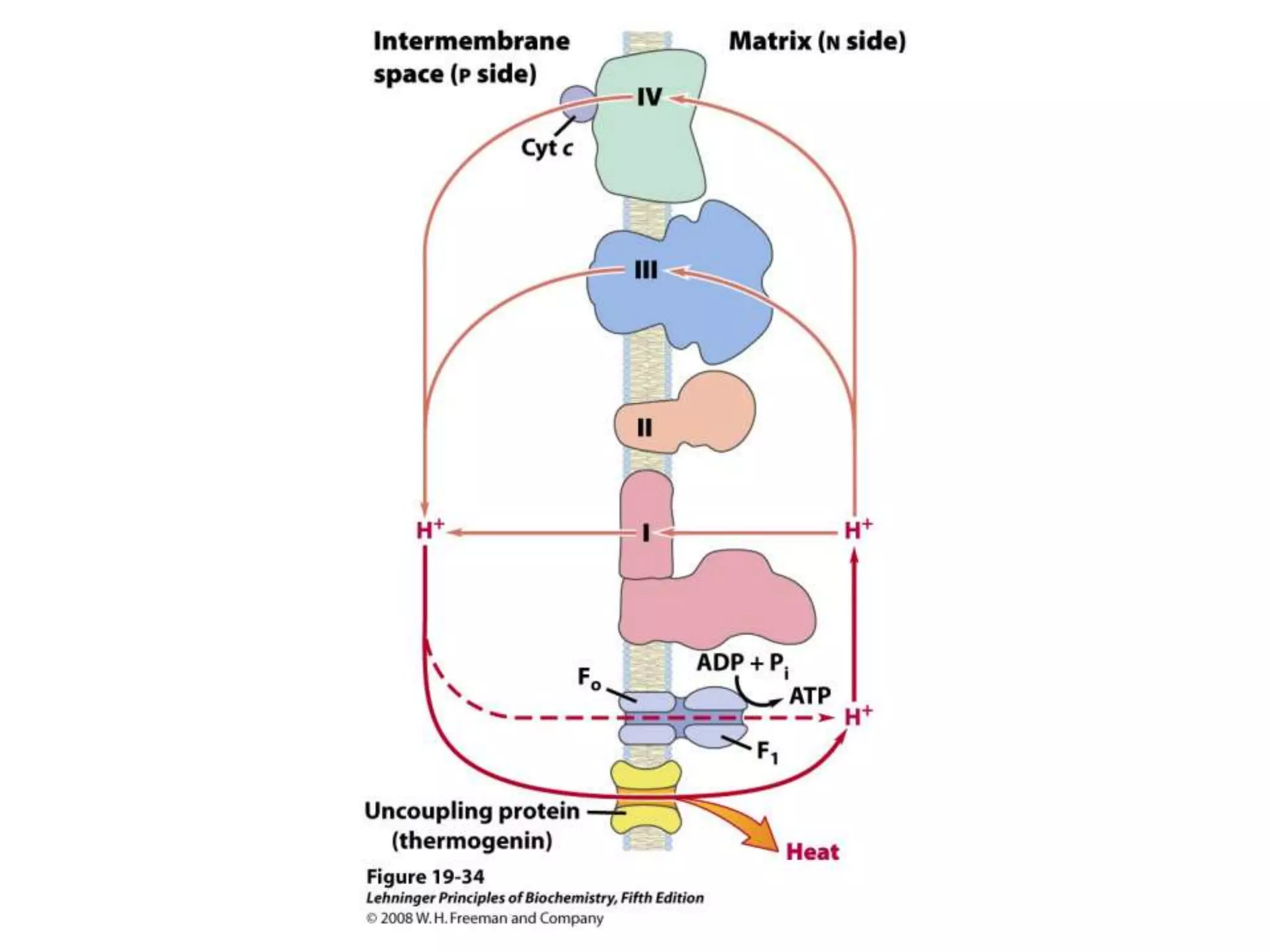
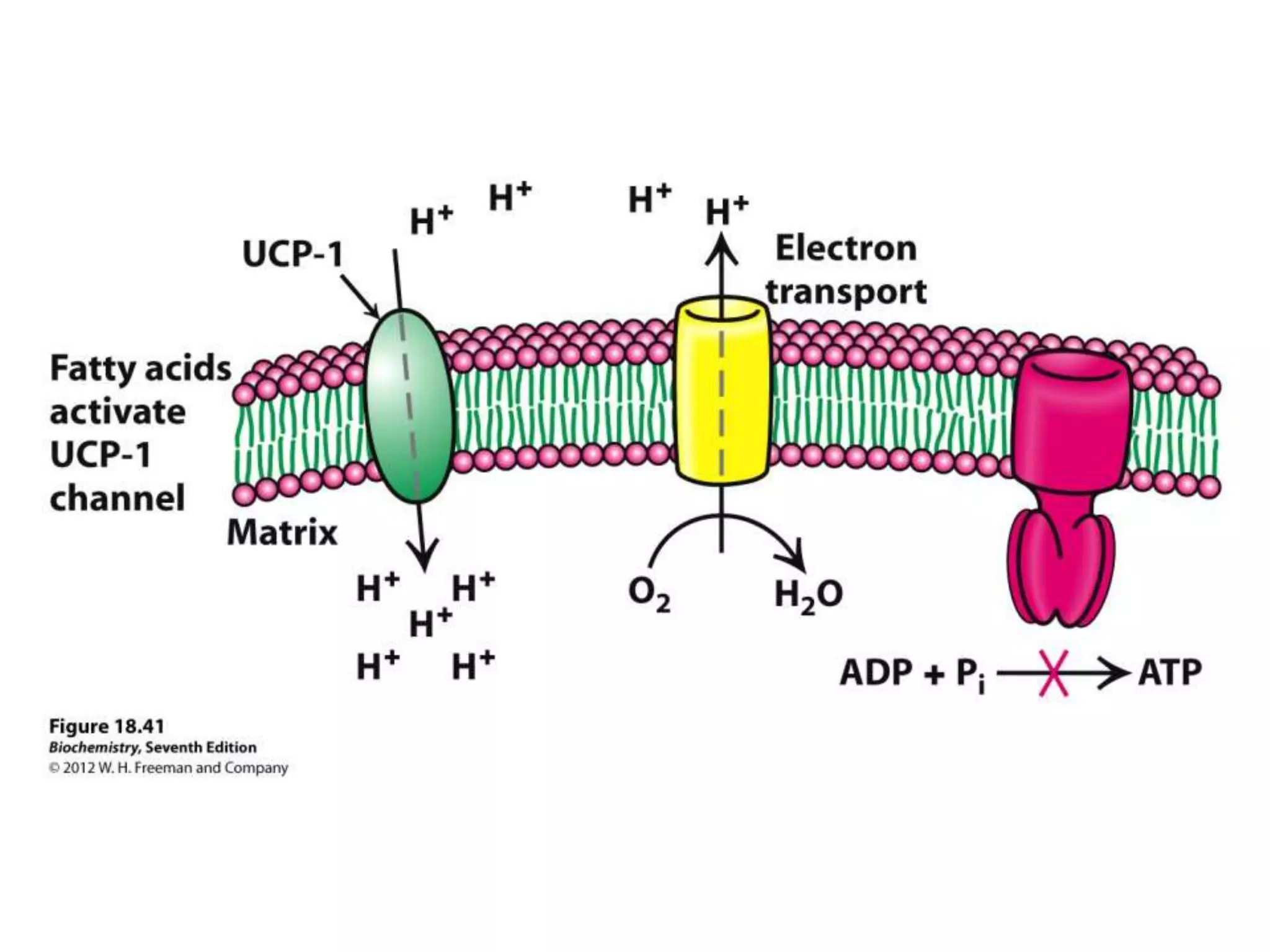
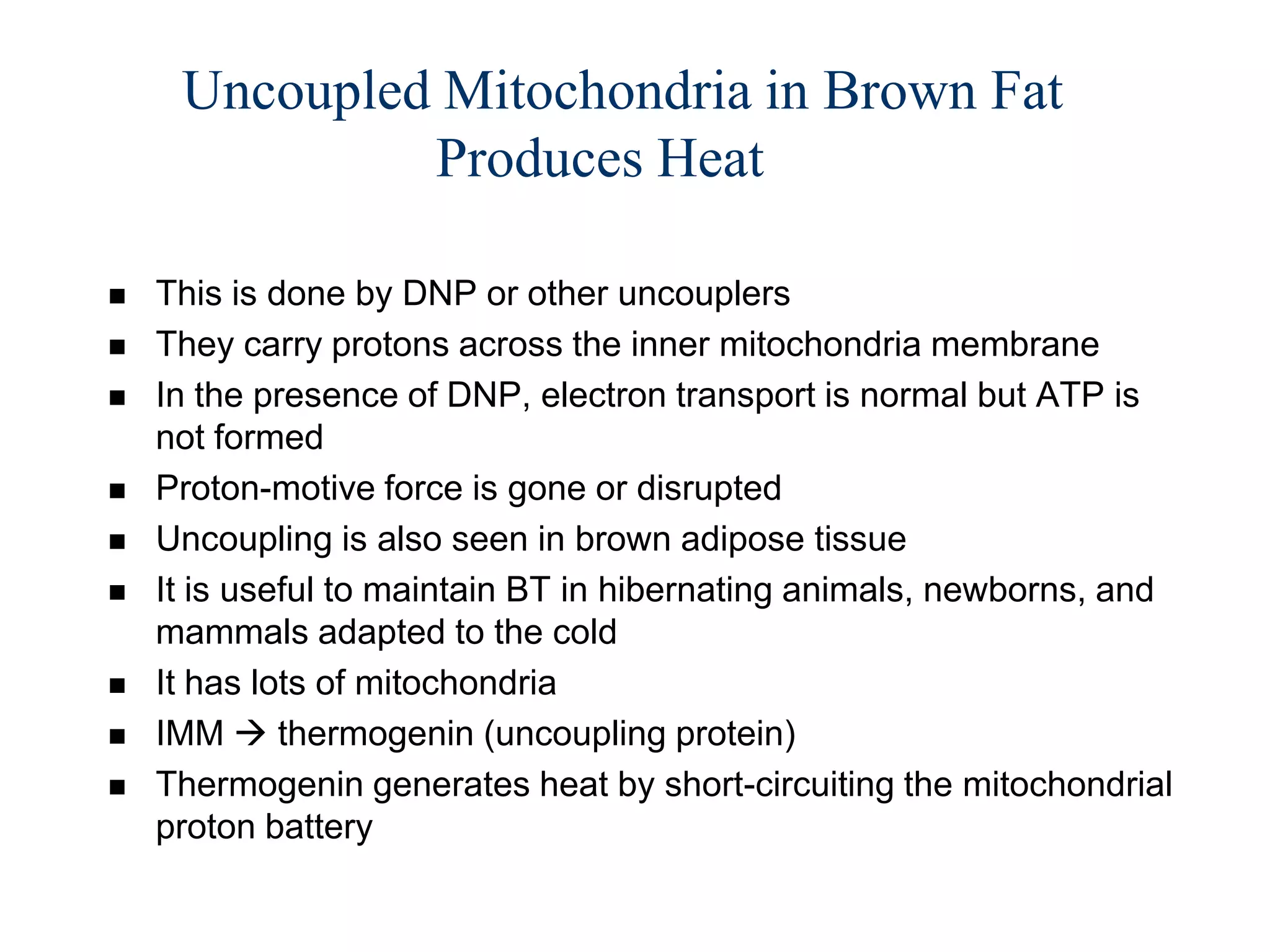
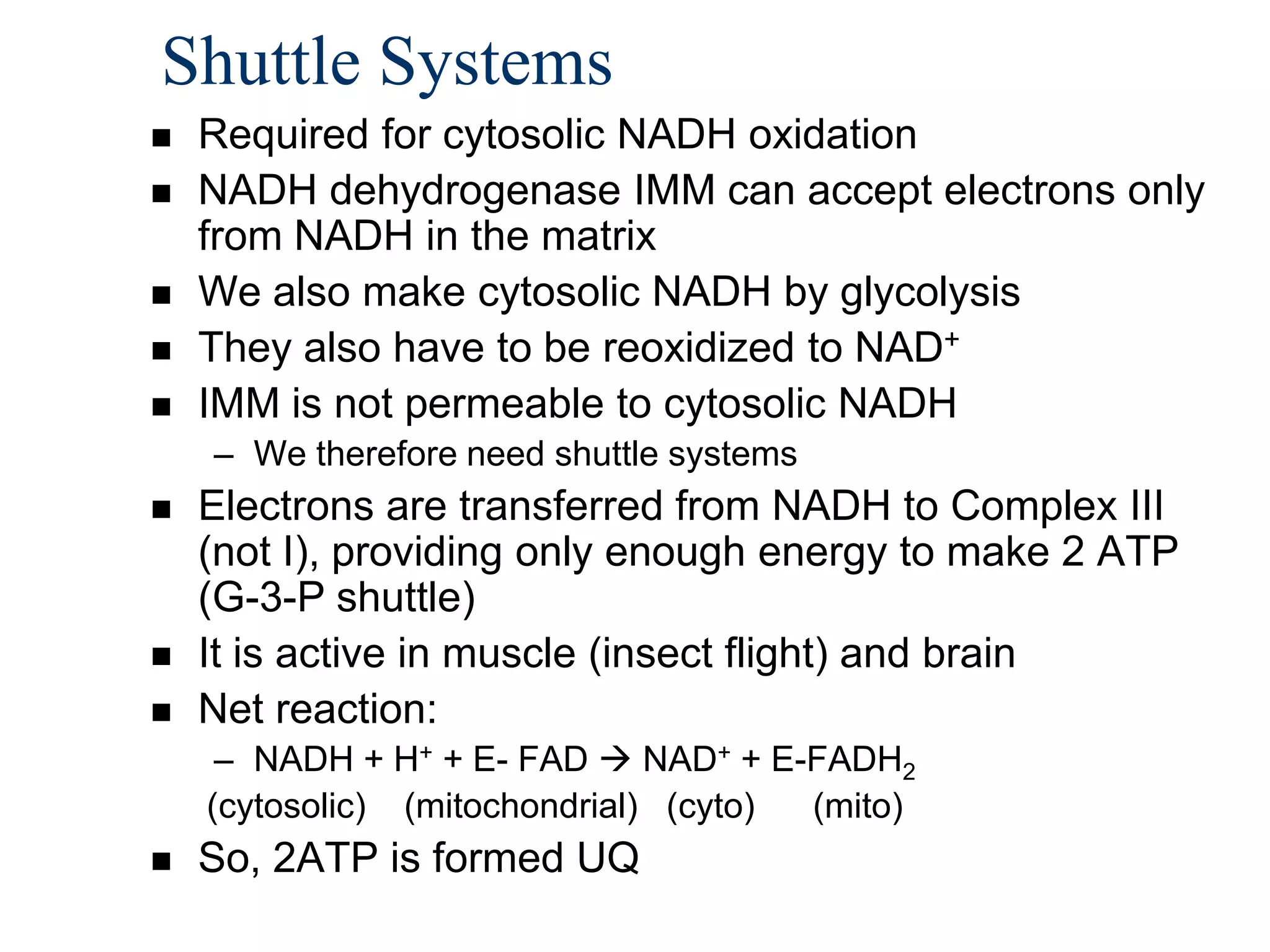
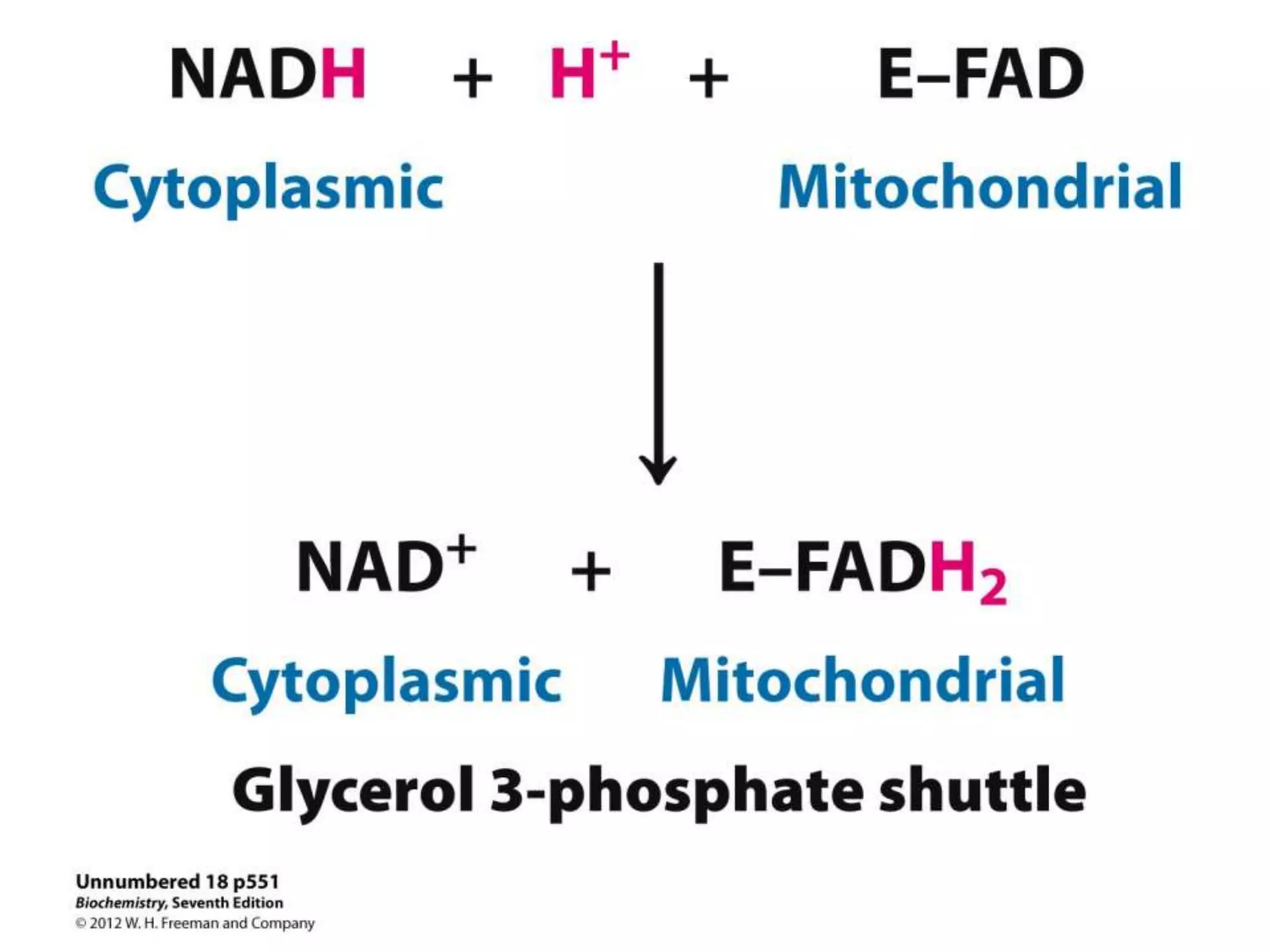
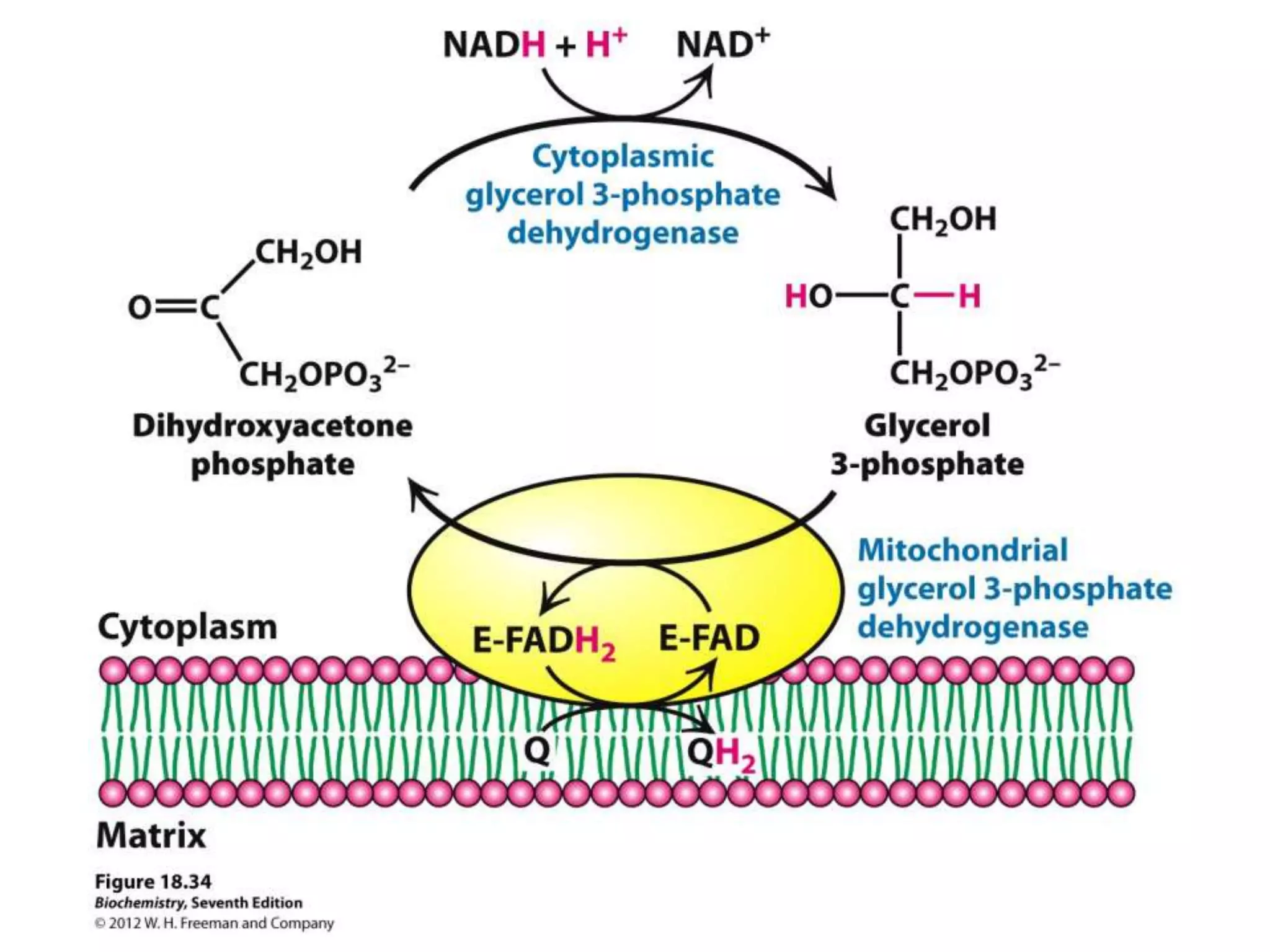
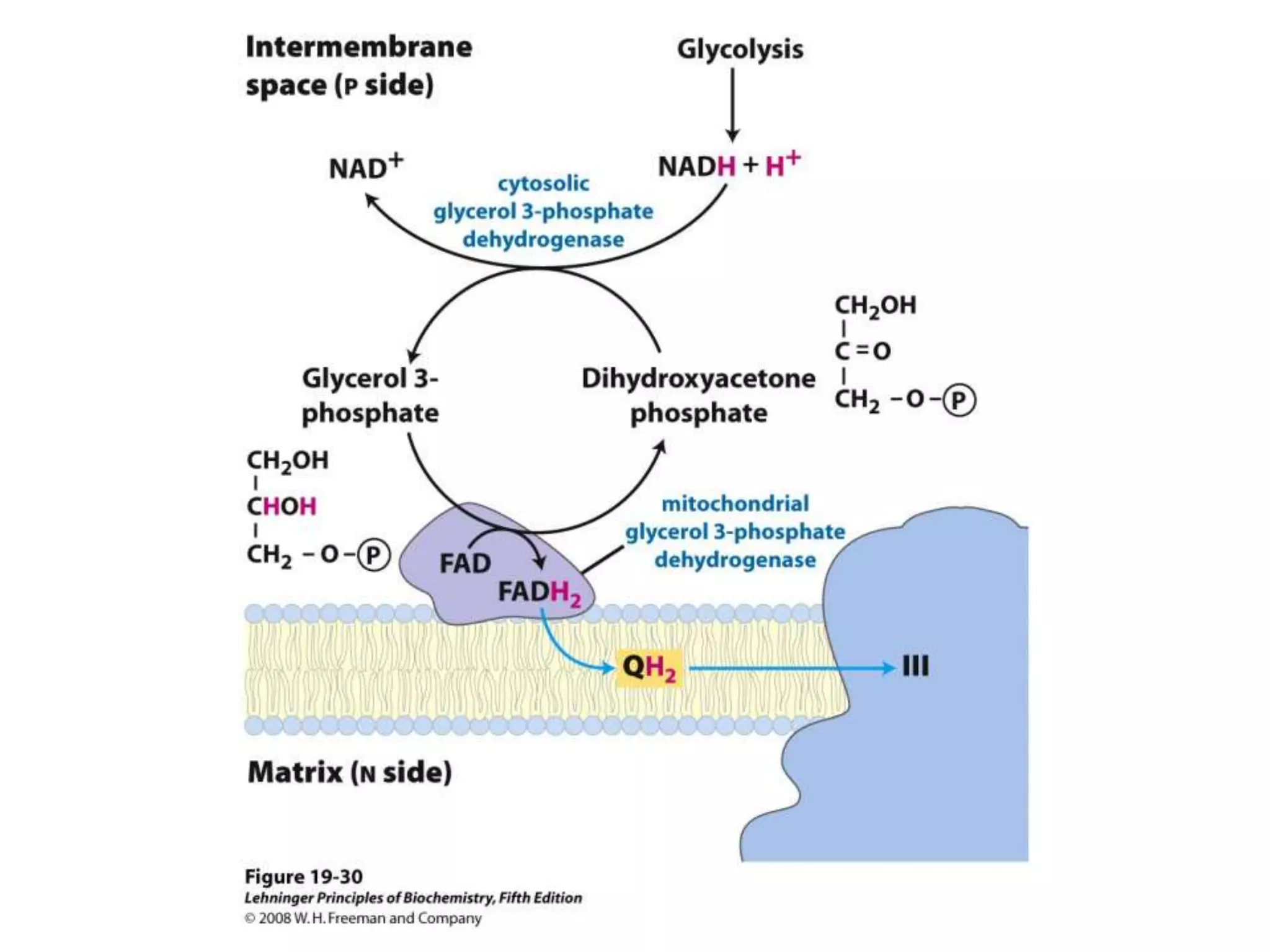
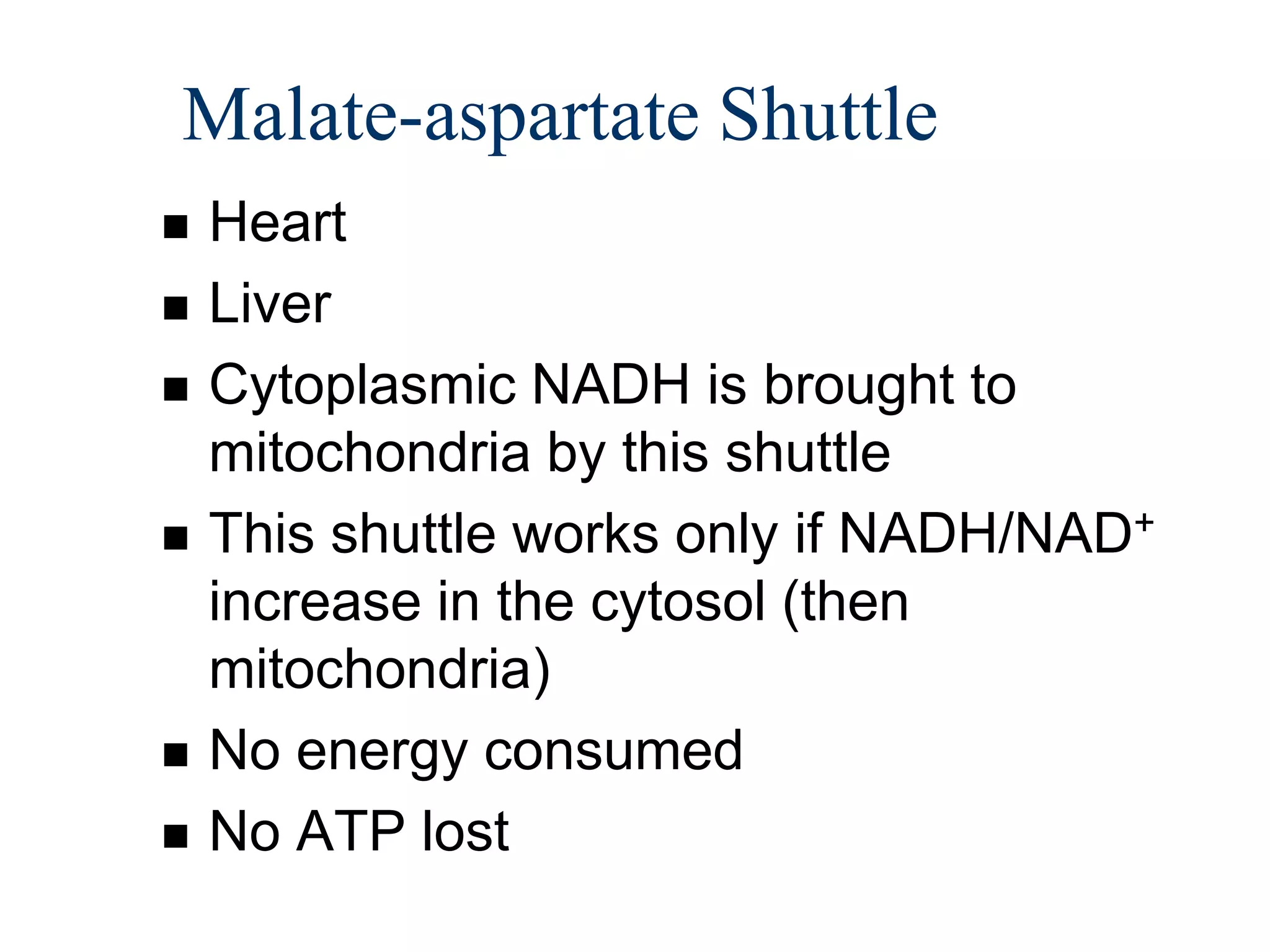
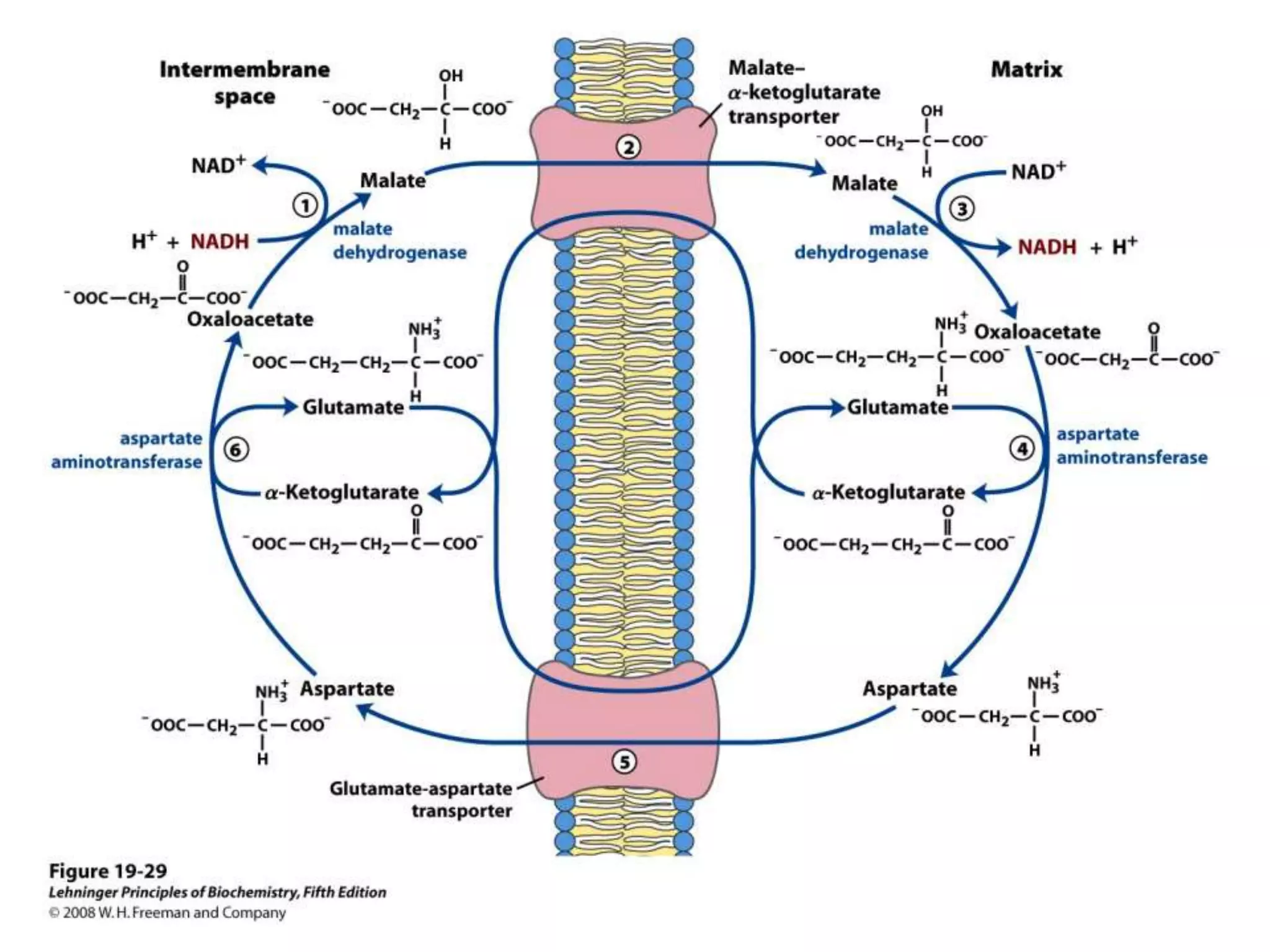
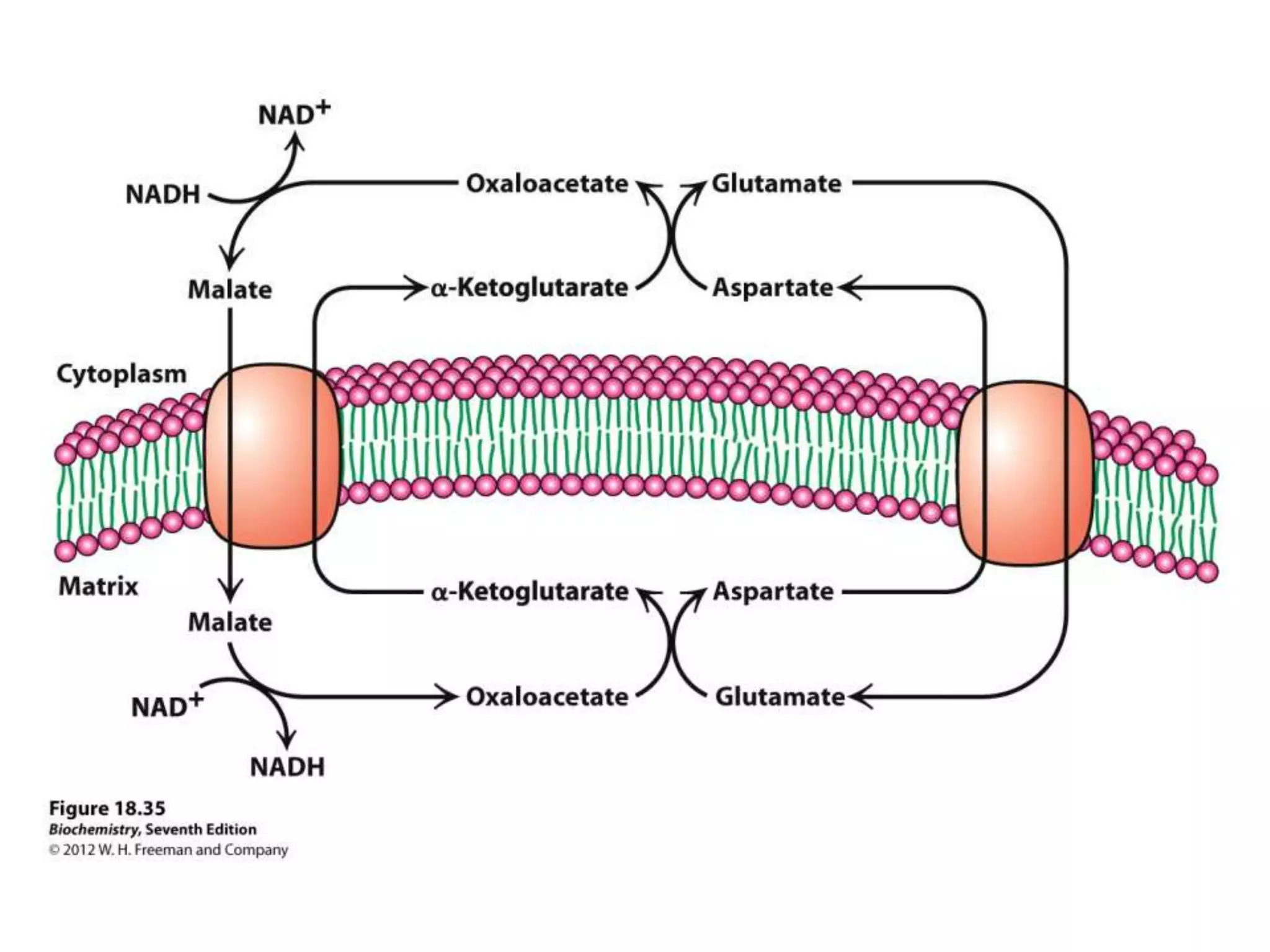
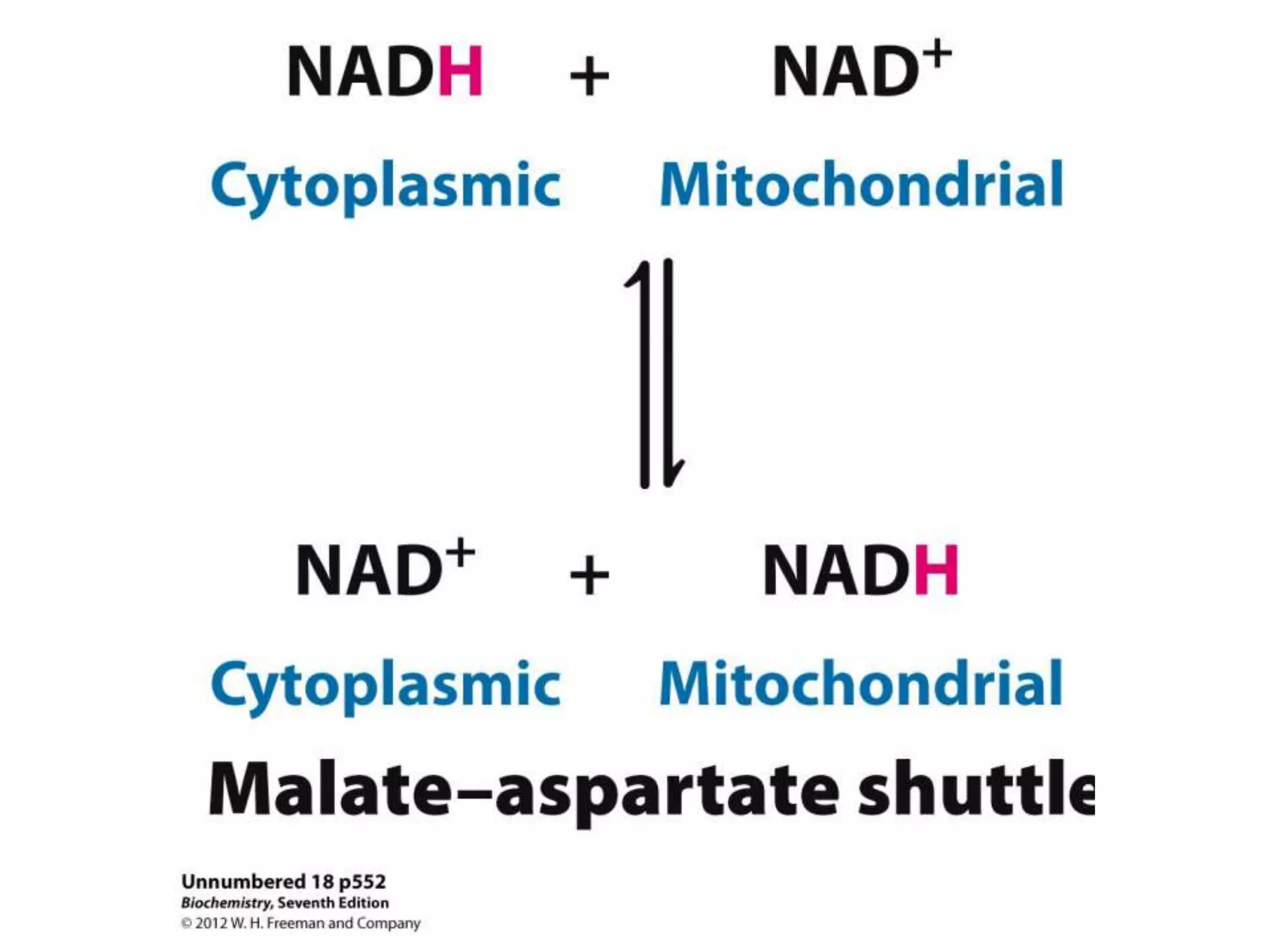
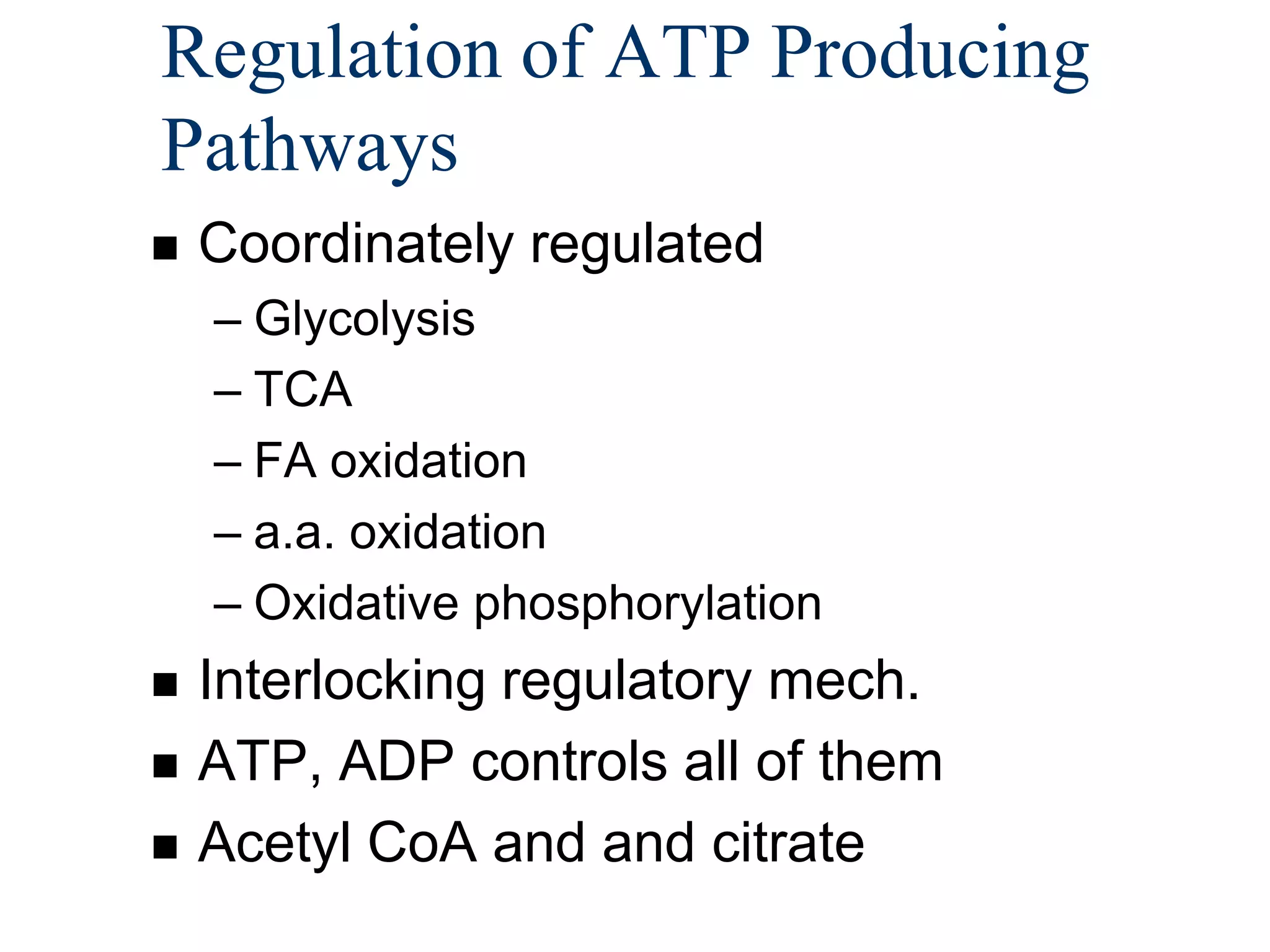
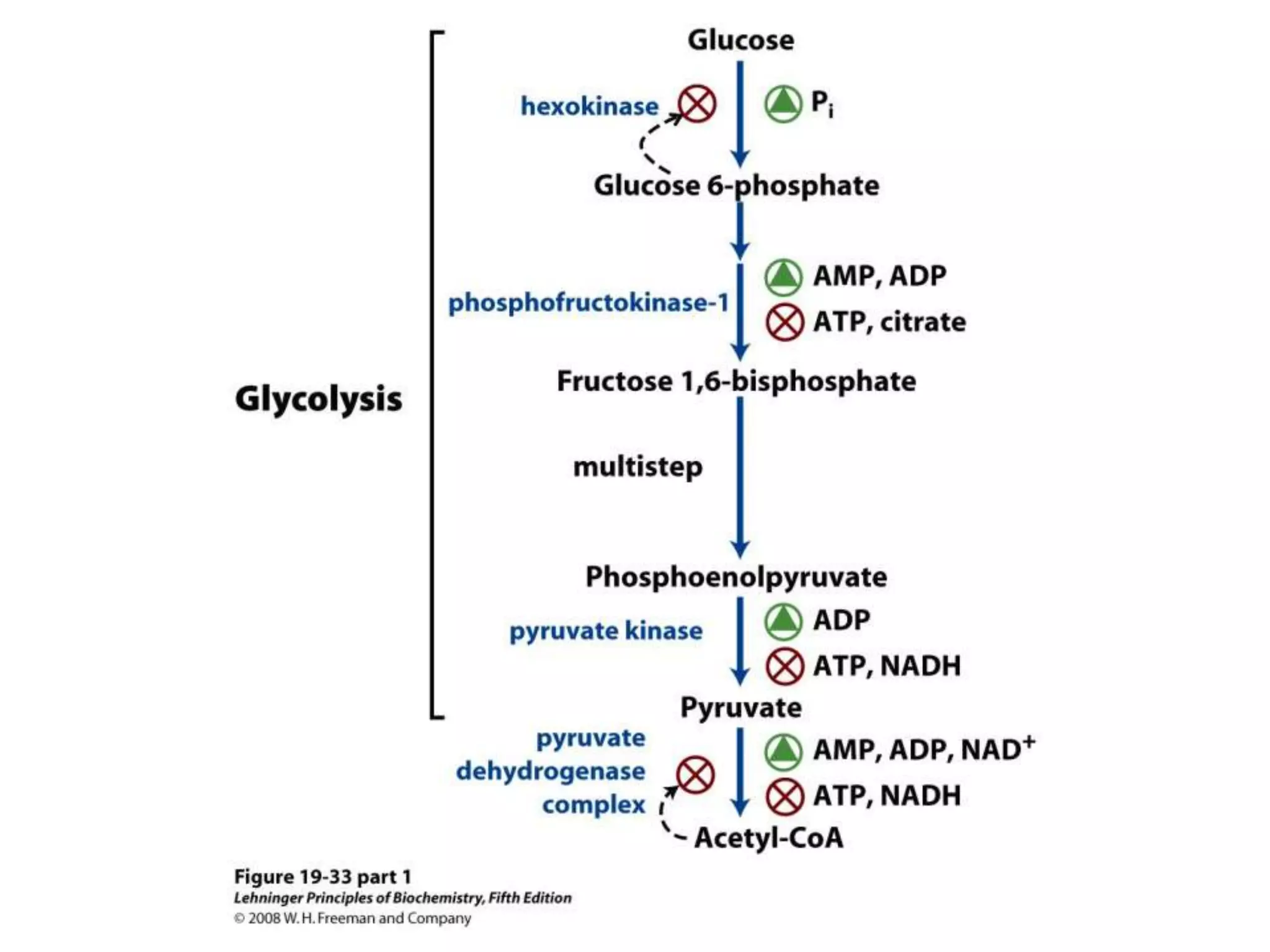
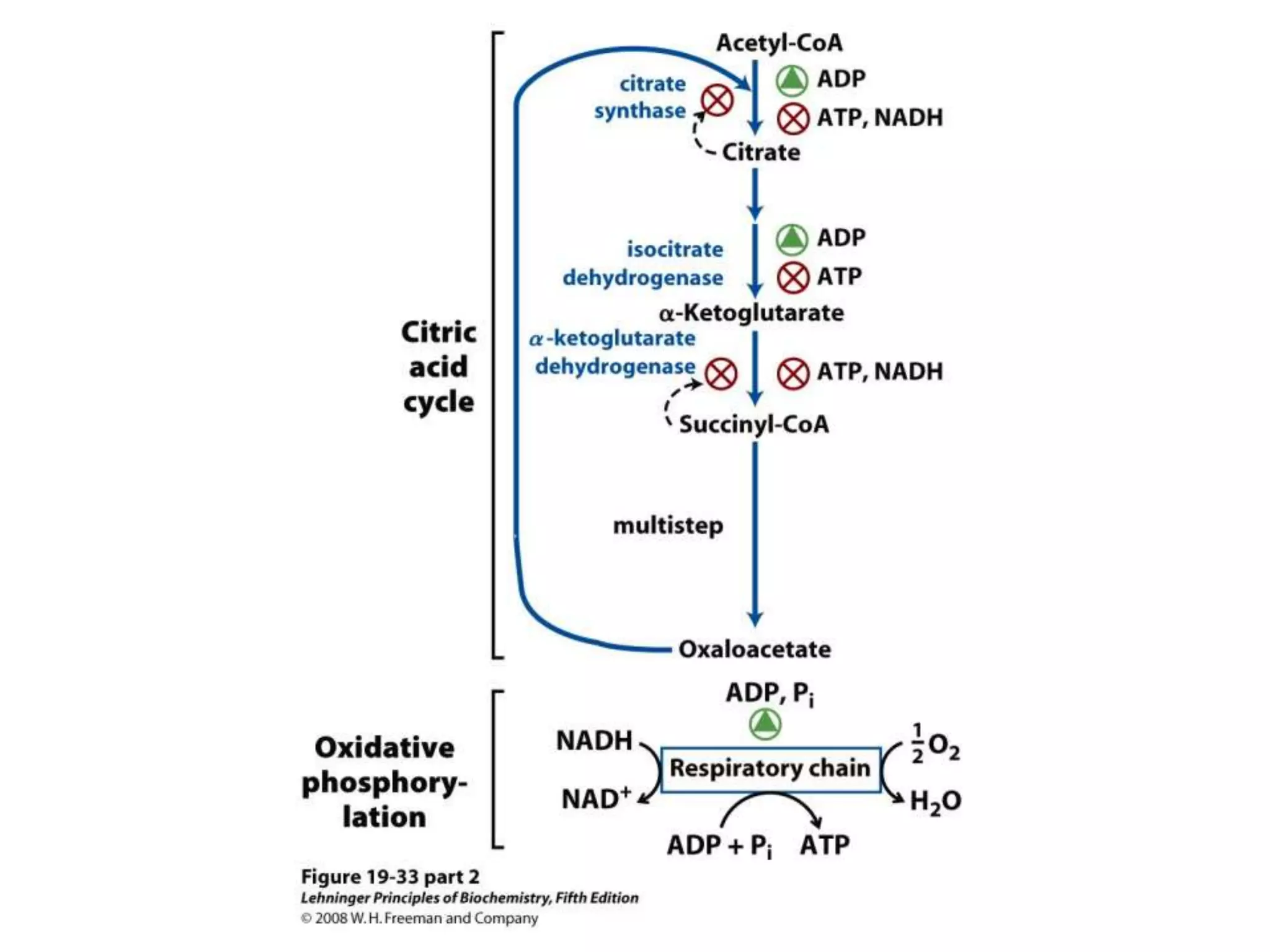
The document summarizes key aspects of oxidative phosphorylation and ATP production in mitochondria. It describes how electrons from NADH and FADH2 are transferred through electron transport chain complexes I-IV, pumping protons out of the mitochondrial matrix. This generates a proton gradient that drives ATP synthase to phosphorylate ADP to ATP. The coupling of electron transport and ATP production via this proton gradient is explained by Mitchell's chemiosmotic theory.






![Chemiosmotic Theory Continued…
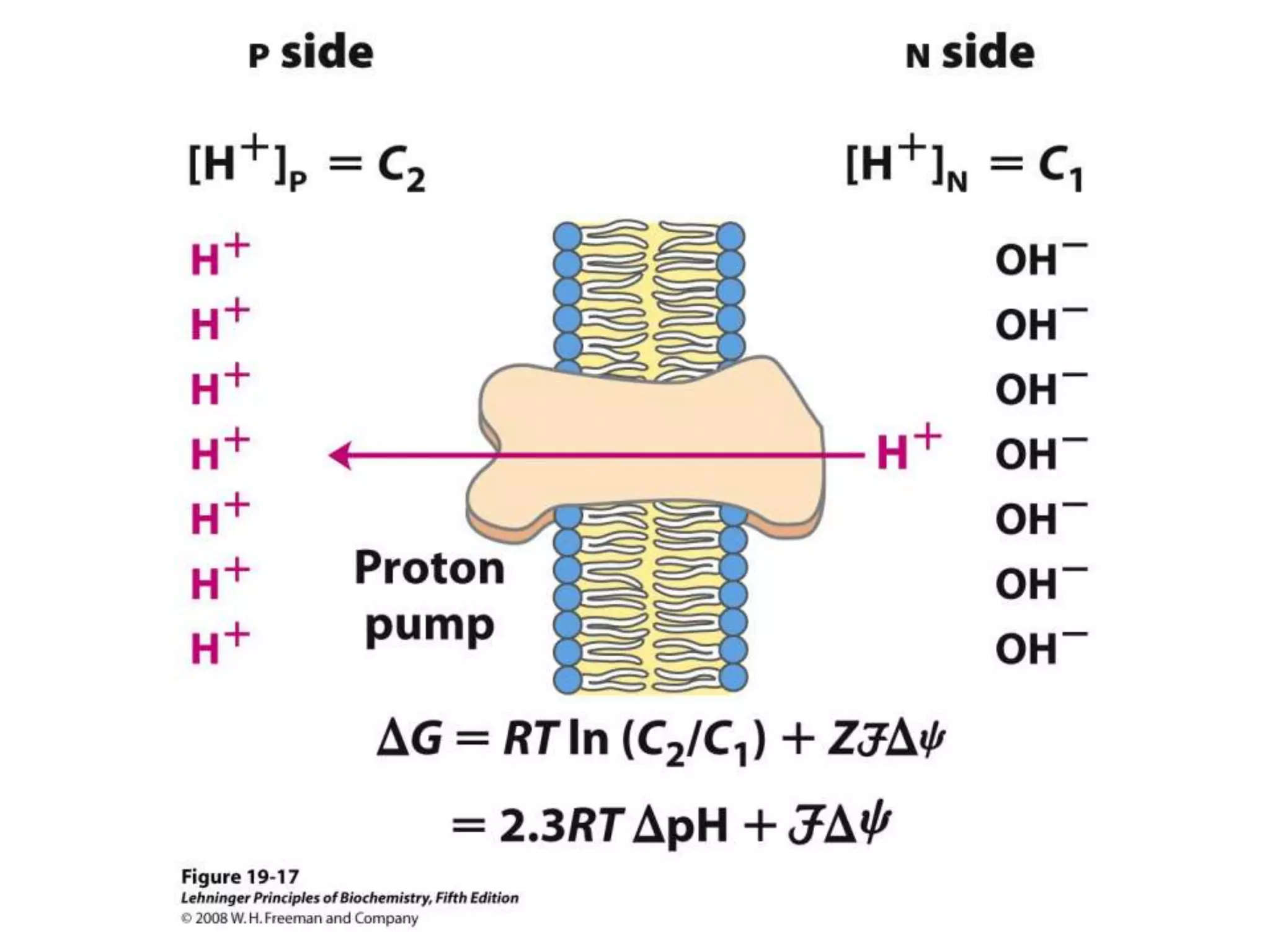
G = RT ln(C2/C1) + ZF
[ + ] [ + ]
When H+ is pumped against electrochemical gradient
G=+
When protons flow back inside, this G becomes
available to do the work!!](https://image.slidesharecdn.com/lec06oxidativep-130314232948-phpapp02/75/Lec06-oxidative-p-61-2048.jpg)
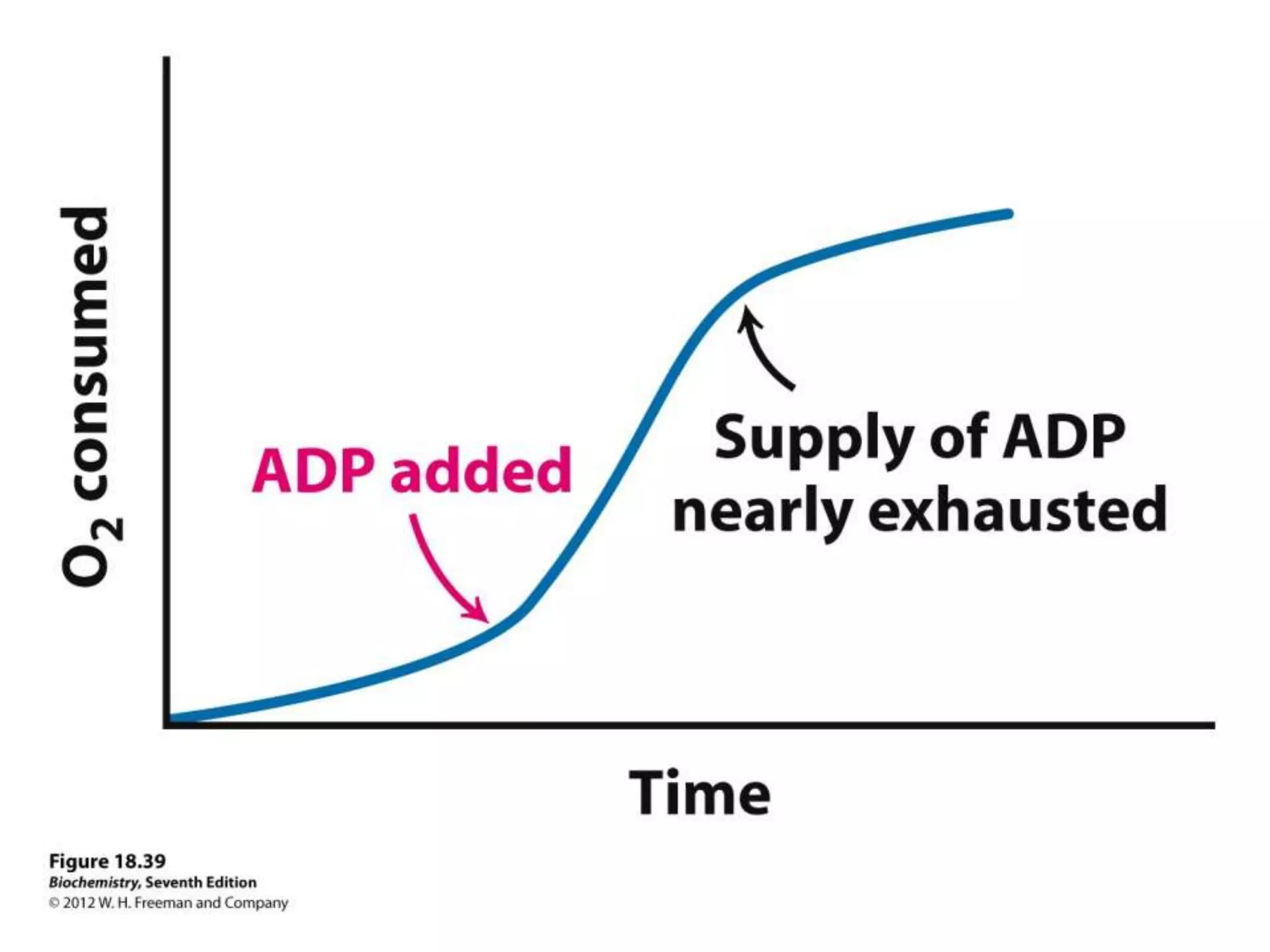
![Regulation of Oxidative Phosphorylation
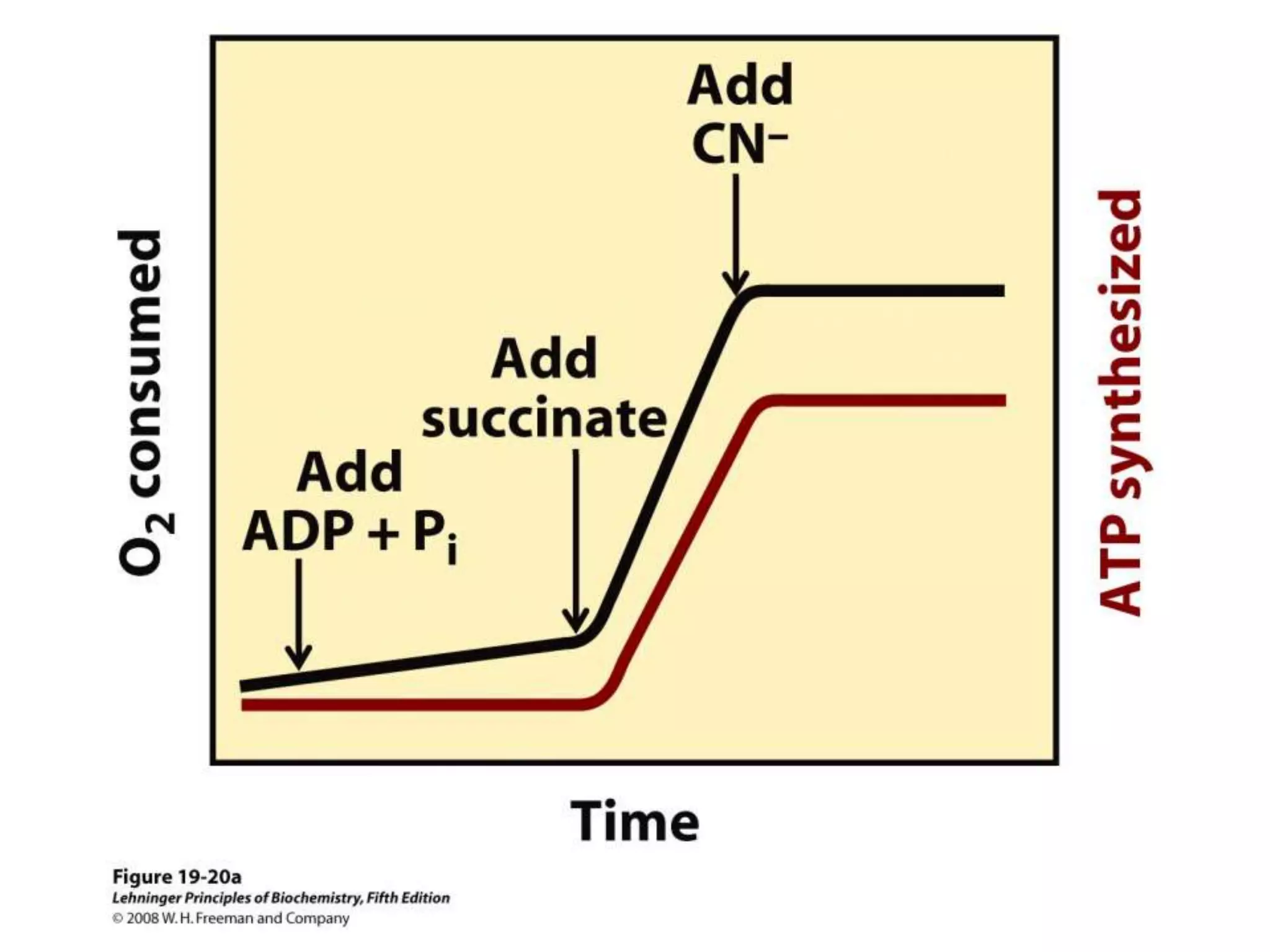
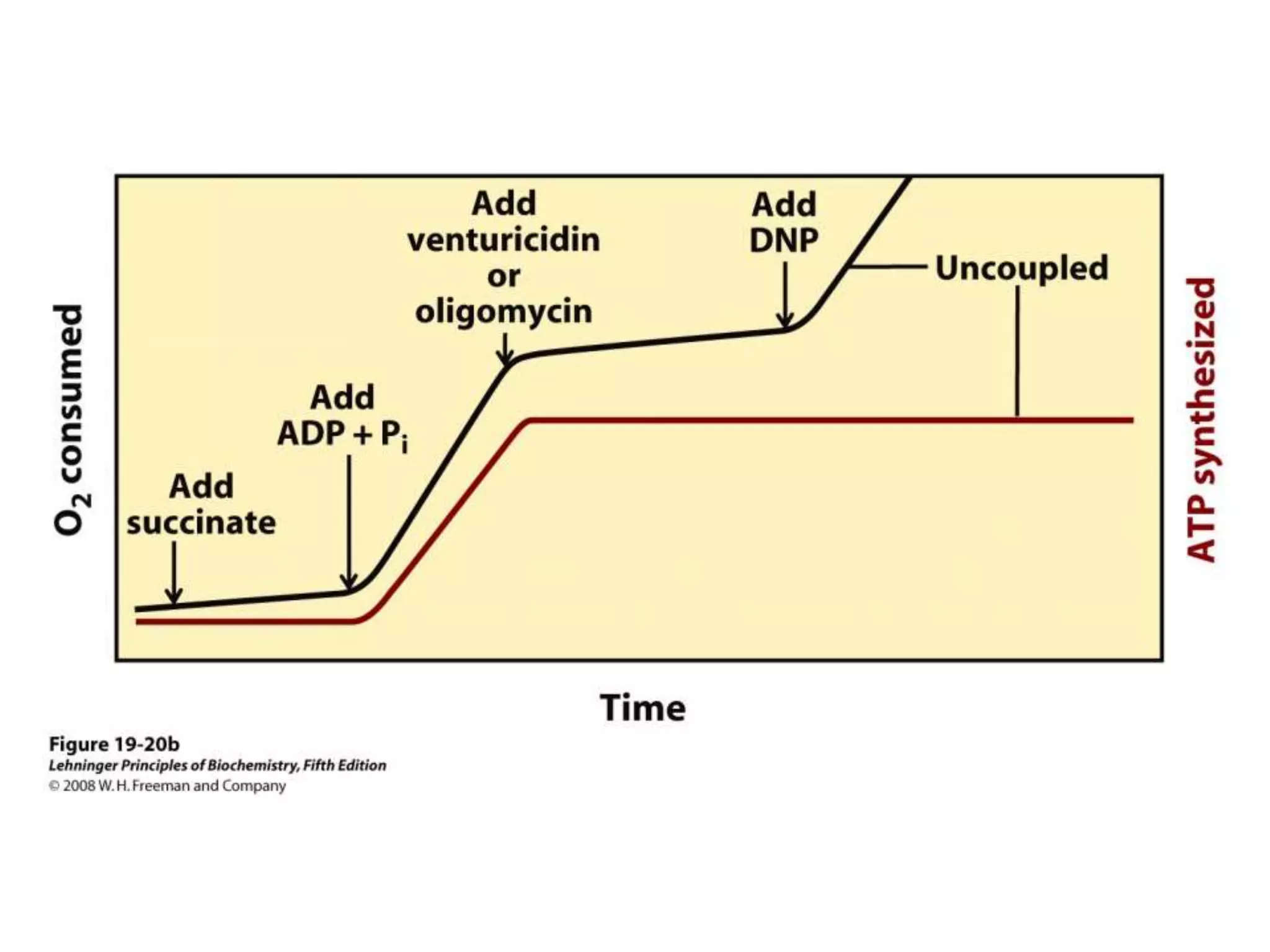
Intracellular [ADP]
If no ADP no ATP
– The dependence of the rate of O2 consumption on the
[ADP] (Pi acceptor) is called “acceptor control”
acceptor control ratio = ADP-induced O2 consumption
O2 consumption without ADP
Mass action ratio:
ATP is high normally
[ADP][Pi]
So, system is fully phosphorylated.
ATP used, ratio decreases, rate of oxidative phosphorylation
increases.](https://image.slidesharecdn.com/lec06oxidativep-130314232948-phpapp02/75/Lec06-oxidative-p-94-2048.jpg)