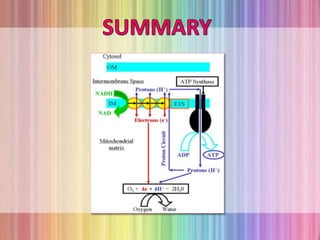
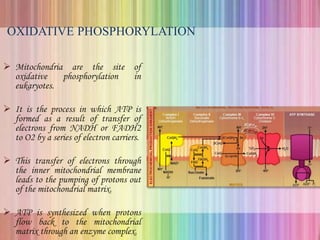
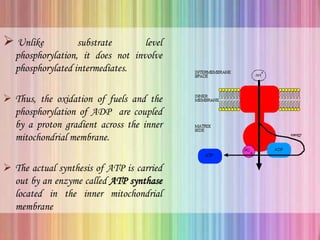
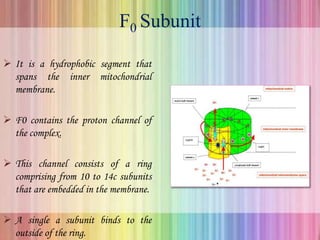
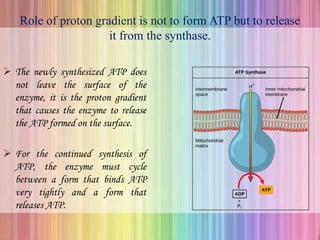
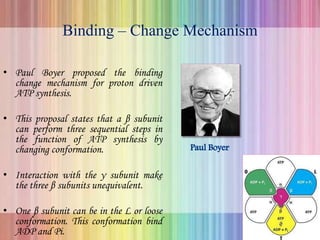
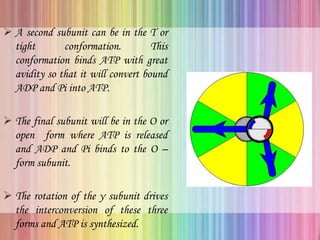
Oxidative phosphorylation occurs in the mitochondria, where ATP is produced through the transfer of electrons from NADH or FADH2 to O2, creating a proton gradient that drives ATP synthesis via ATP synthase. The process is regulated by cellular energy demands, with ADP levels influencing the rate of respiration and ATP regeneration. Under hypoxic conditions, a protein inhibitor named IF1 prevents ATP hydrolysis, maintaining ATP production by blocking ATP synthase from reversing its function.


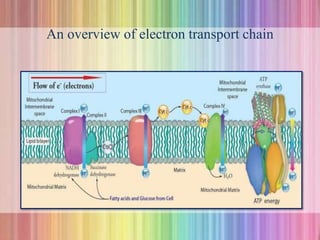

![ In actively respiring mitochondria,
the actions of many dehydrogenases
keep the actual [NADH]/[NAD]
ratio well above unity, and the real
free energy change for the reaction is
substantially greater (more negative)
than -220 kJ/mol.
Much of this energy is used to
pump protons out of the matrix.
For each pair of electrons
transferred to O2, four protons are
pumped out by Complex I, four by
Complex III and two by Complex
IV.](https://image.slidesharecdn.com/oxidativephosphorylation-170722064648/85/Oxidative-phosphorylation-7-320.jpg)







![REGULATION OF OXIDATIVE PHOSPHORYLATION
Oxidative phosphorylation is regulated by
cellular energy needs.
o The intracellular [ADP] and the mass action ratio
([ADP]/[ATP][Pi]) are measures of cells energy status.
o Normally this ratio is very high, so the ATP-ADP system is
almost fully phosphorylated.
o When the rate of some energy requiring process increases, the rate
of breaking down of ATP to ADP and Pi increases, lowering the
mass-action ratio.
o When more ADP is available for oxidative phosphorylation, the
rate of respiration increases, causing regeneration of ATP.](https://image.slidesharecdn.com/oxidativephosphorylation-170722064648/85/Oxidative-phosphorylation-27-320.jpg)