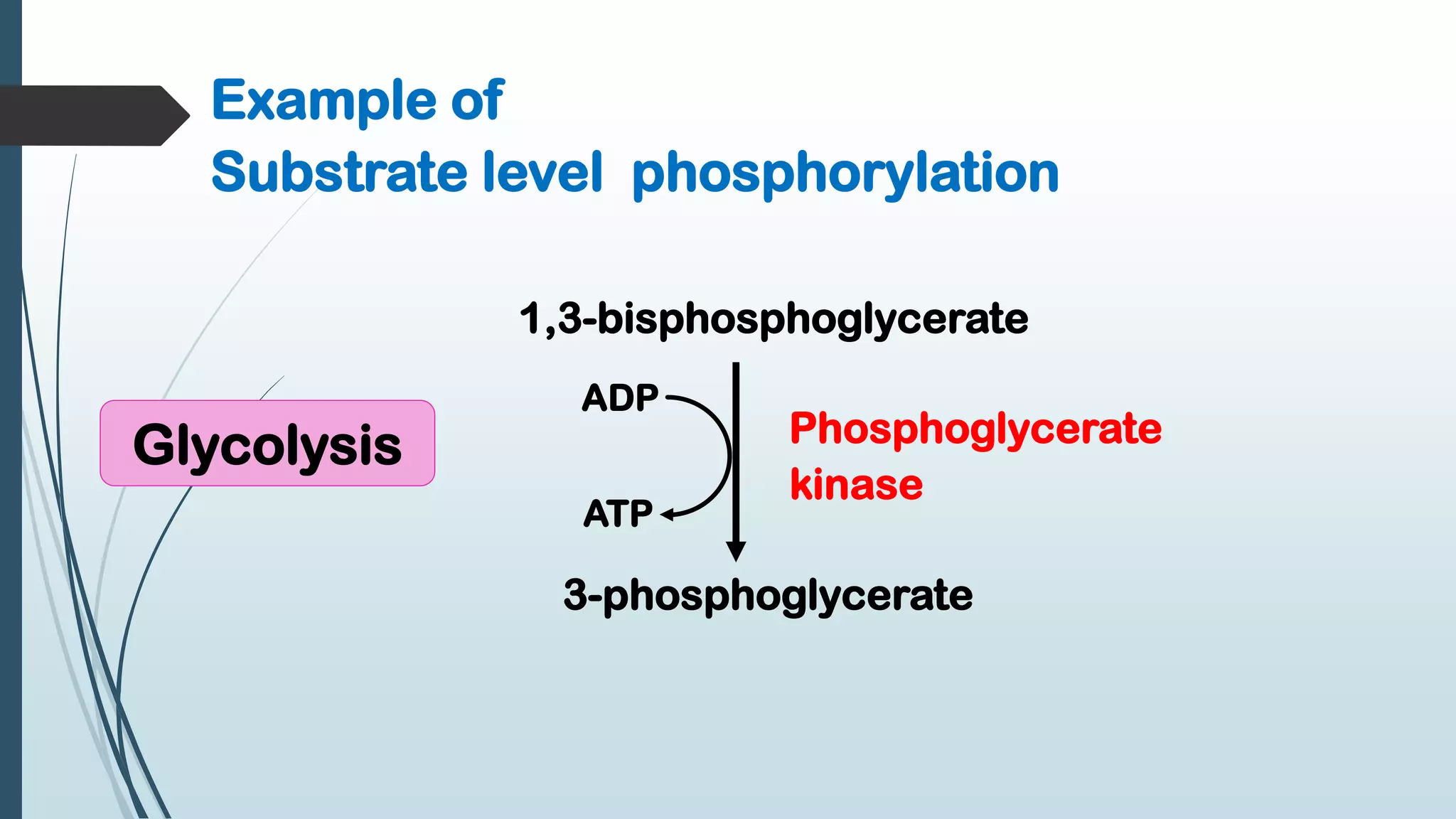
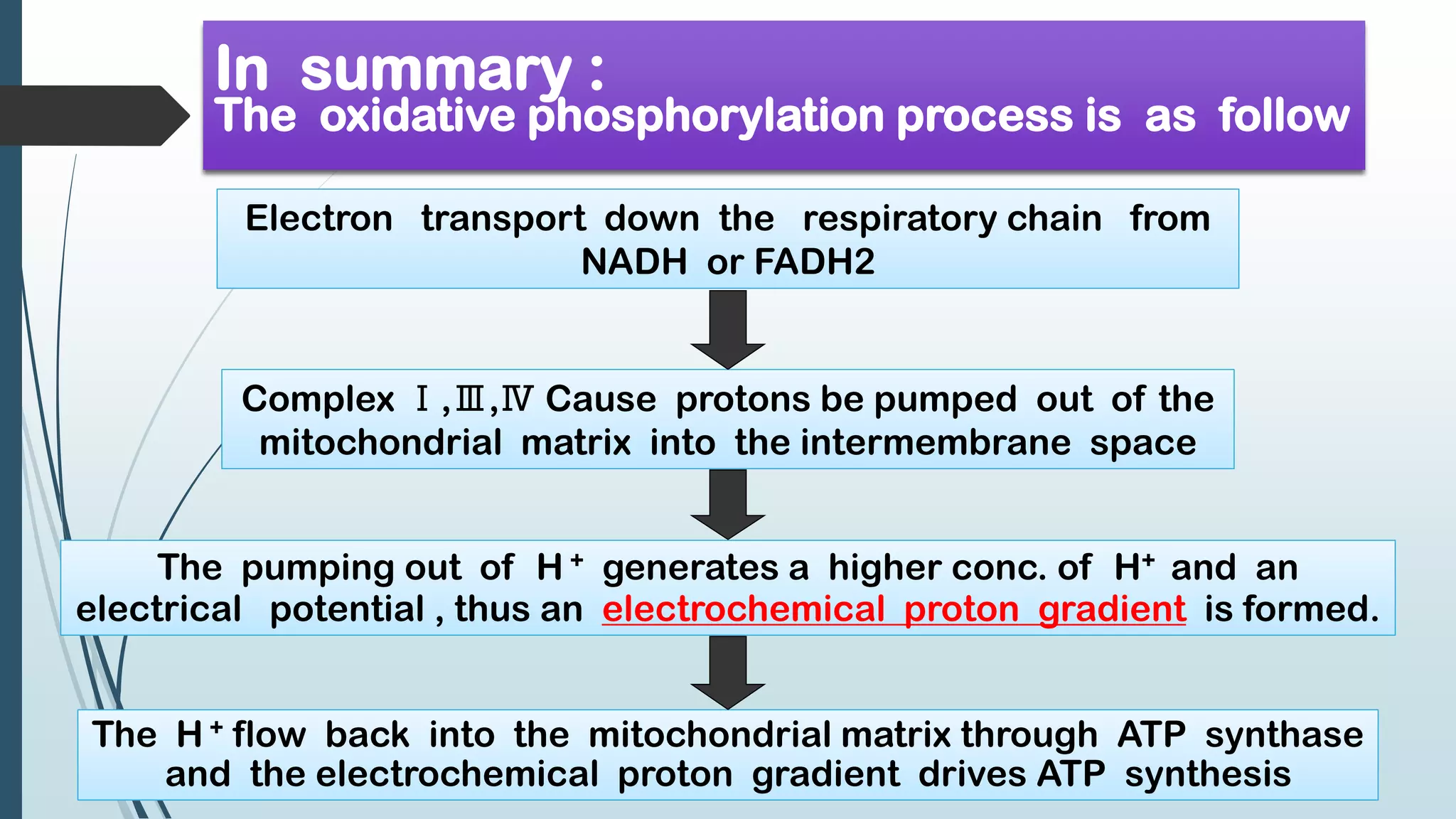
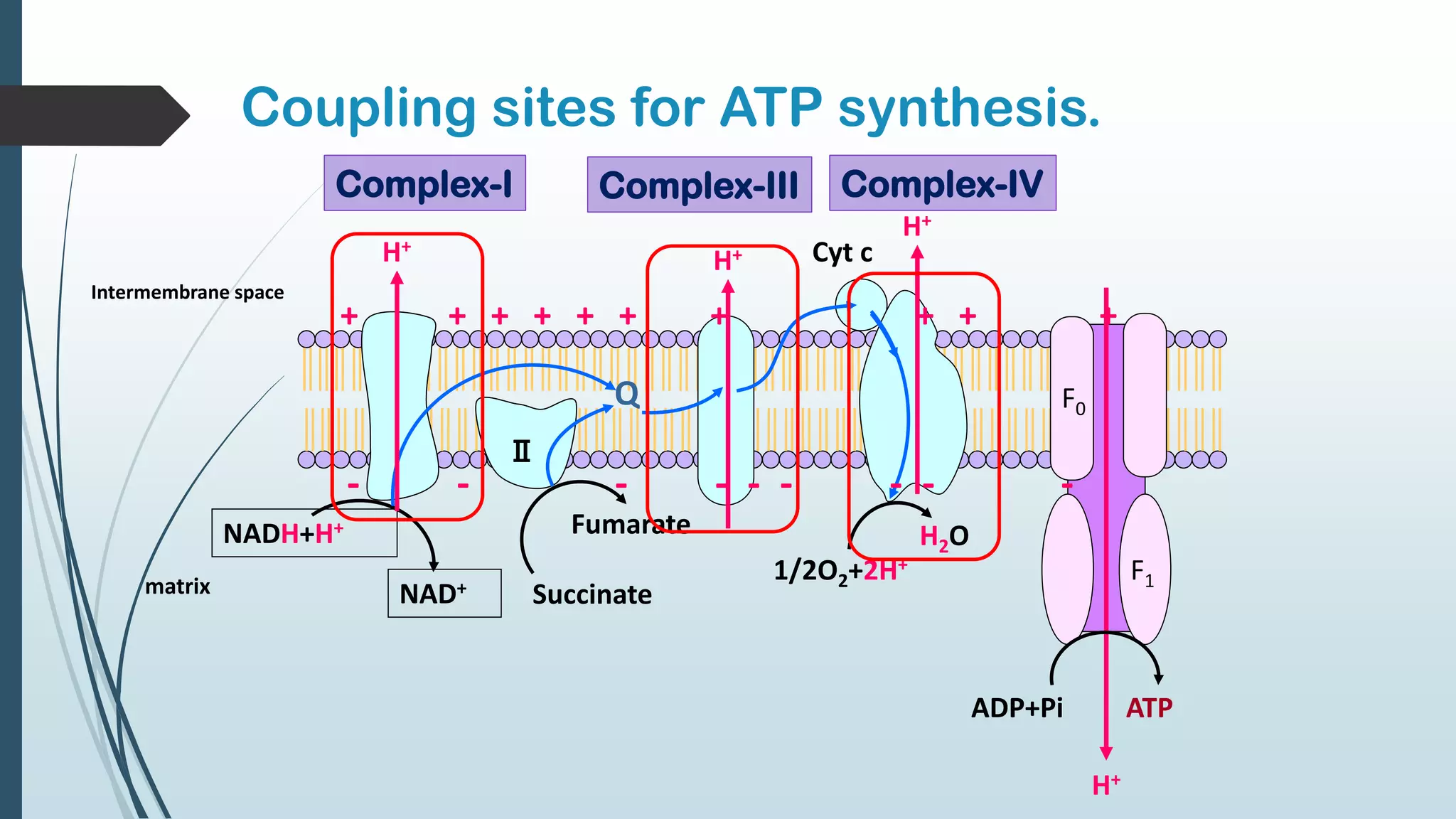
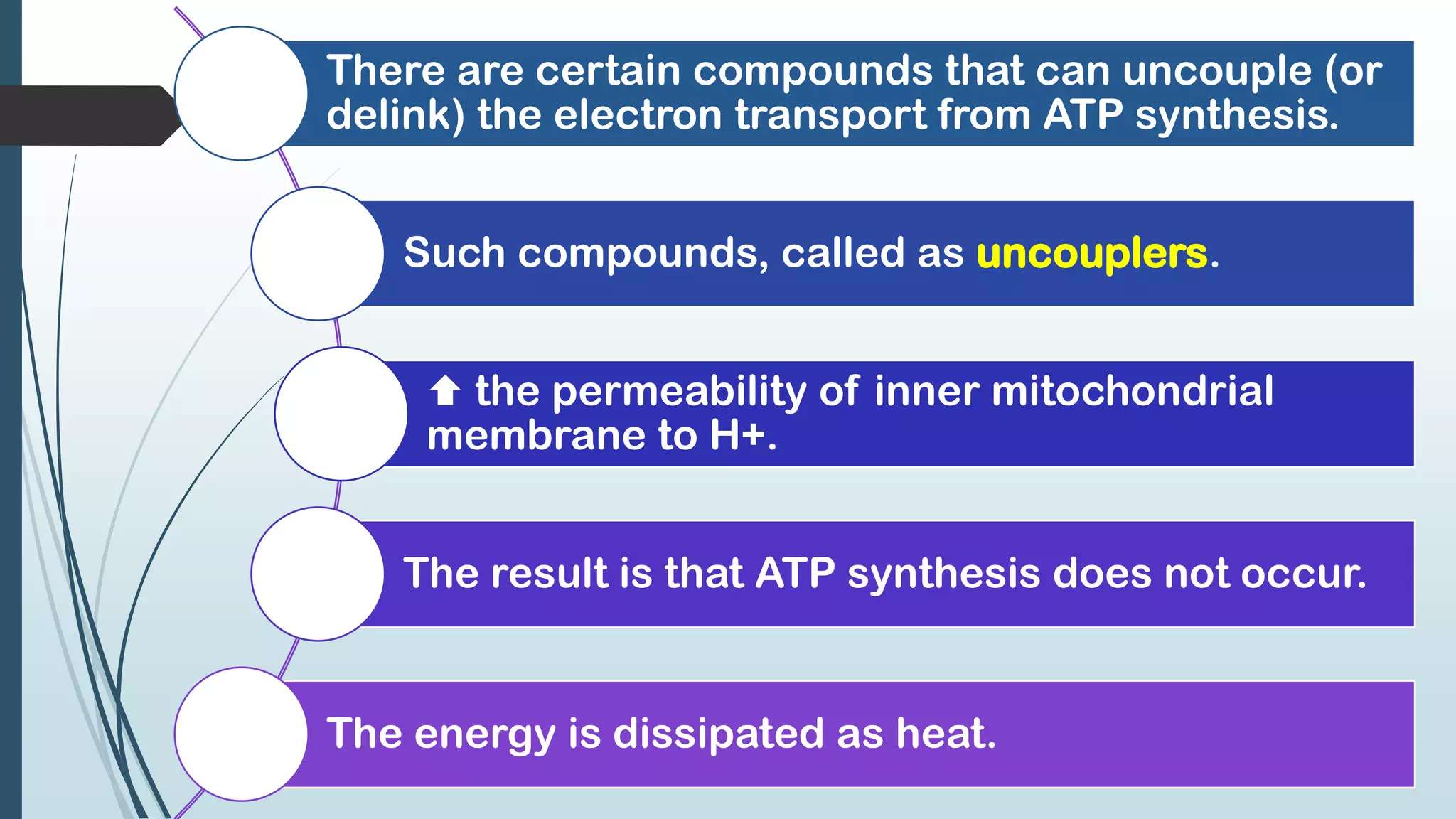
The document discusses the processes of oxidative phosphorylation and substrate-level phosphorylation in ATP synthesis, primarily occurring in the mitochondria. It explains the roles of the electron transport chain and proton gradients in ATP generation, detailing hypotheses including chemiosmotic theory and experimental findings on proton leakage affecting ATP production. Additionally, it covers the mechanisms of uncouplers, ATP synthesis regulation by ADP and ATP levels, and the shuttle systems for transporting NADH into mitochondria.