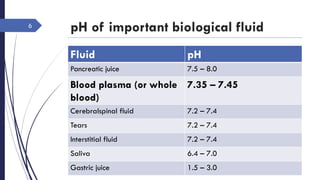
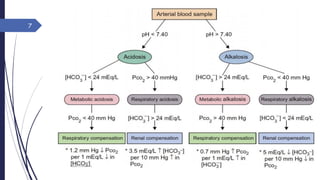
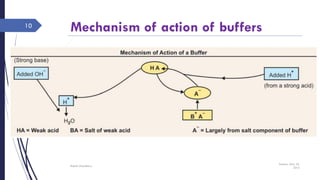
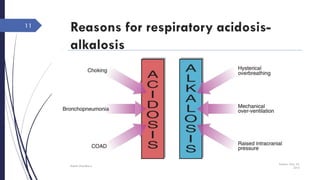
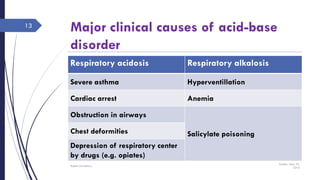
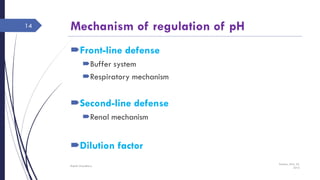
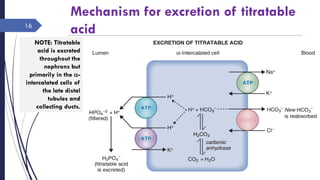
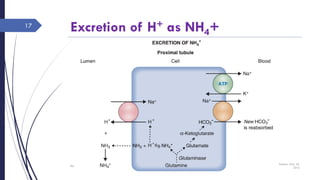
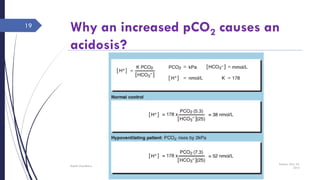
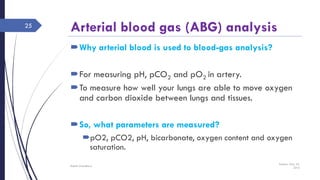
This document discusses acid-base disorders including acidosis and alkalosis. It defines metabolic and respiratory acidosis and alkalosis based on primary disturbances in bicarbonate or pCO2 levels. Compensatory responses between the respiratory and renal systems are described. Causes, examples, and management of respiratory acidosis and alkalosis are provided. Arterial blood gas analysis is explained as a tool to measure acid-base levels and oxygen status in patients with conditions like lung disease. One case presented is identified as chronic respiratory acidosis based on elevated pCO2 and bicarbonate levels.


![Disturbance of acid-base
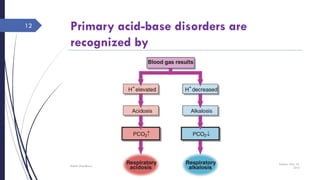
Metabolic: Primary disturbance is in [HCO3-]
[HCO3-] Metabolic acidosis
[HCO3-] Metabolic alkalosis
Respiratory: Primary disturbance is in pCO2
pCO2 Respiratory acidosis, Cause: hypoventillation
pCO2 Respiratory alkalosis, Cause: hyperventillation
Sunday, May 22,
2016
Rajesh Chaudhary
3](https://image.slidesharecdn.com/bloodphregulationnew2016-160522121906/85/Blood-ph-regulation-new-2016-3-320.jpg)









![Apatient has the following arterial
blood values pH, 7.33; [HCO3-], 36
mEq/L; pCO2, 70 mm Hg. What is the
patient’s acid-base disorder? Is it acute or
chronic?
Comment on the case.
Sunday, May 22,
2016
Rajesh Chaudhary
27
Reference ranges
1. pH: 7.37-7.42
pCO2: 40 mmHg
2. [HCO3-]: 24 mEq/L](https://image.slidesharecdn.com/bloodphregulationnew2016-160522121906/85/Blood-ph-regulation-new-2016-27-320.jpg)
