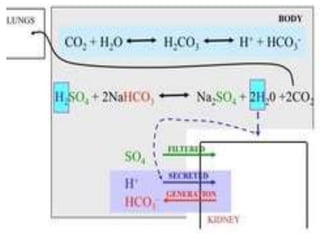
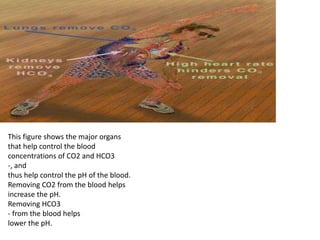
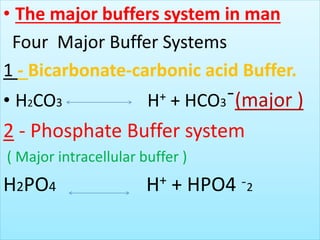
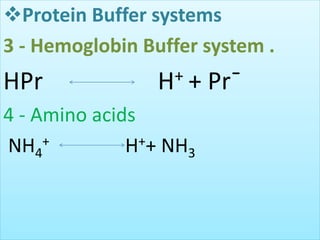
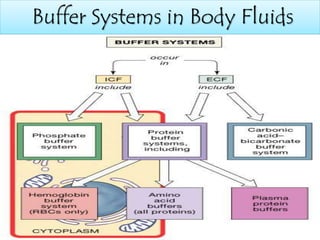
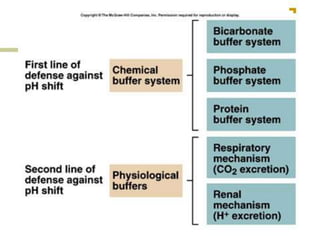
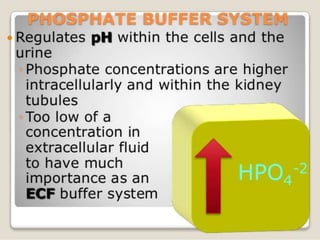
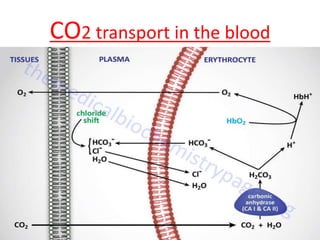
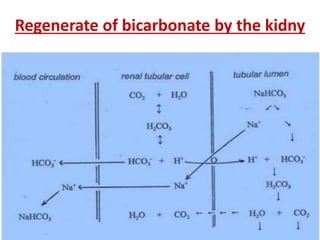
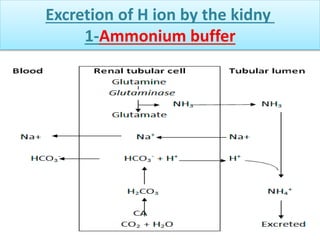
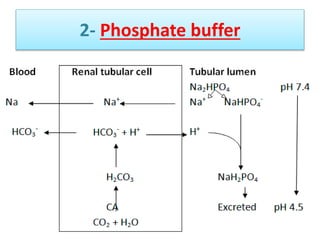
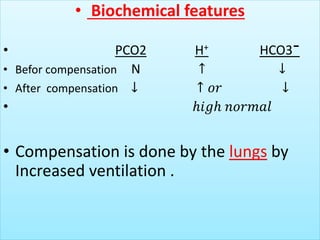
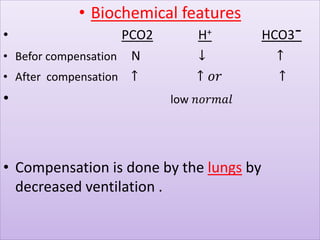
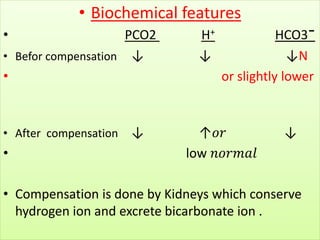
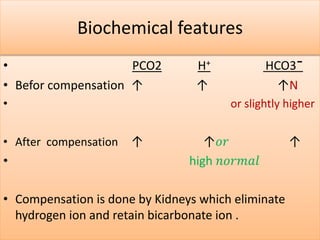
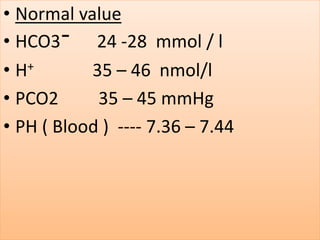
The document discusses acid-base balance and buffer systems in the human body. It describes how the body maintains a slightly basic pH between 7.35-7.45 through various systems like the lungs, kidneys, and important buffer systems. The major buffer system is the bicarbonate-carbonic acid buffer system, which functions to instantly buffer changes in pH. Disorders that disrupt acid-base balance like metabolic acidosis, alkalosis, respiratory acidosis and alkalosis are explained along with their causes and compensatory mechanisms.