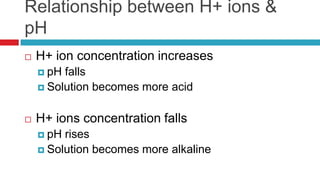
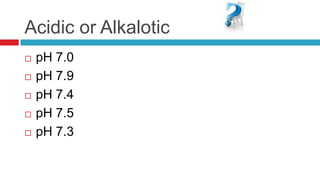
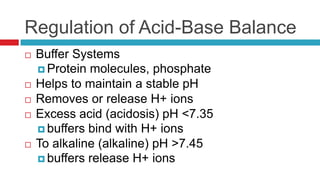
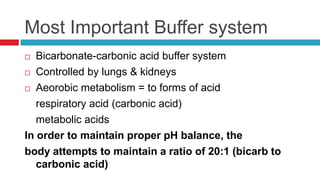
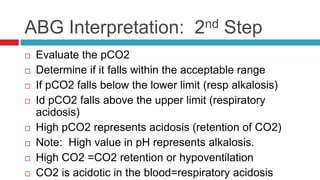
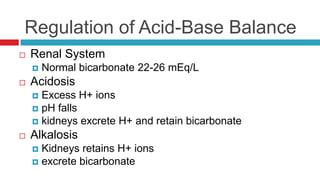
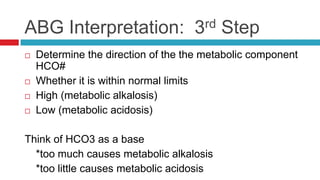
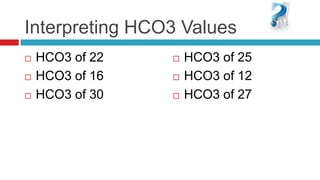
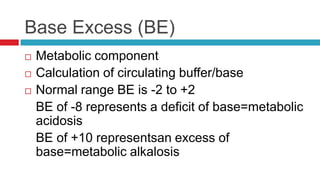
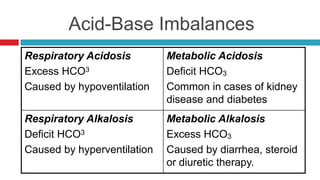
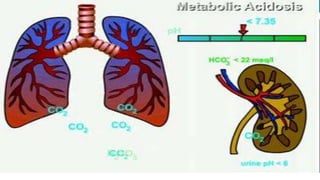
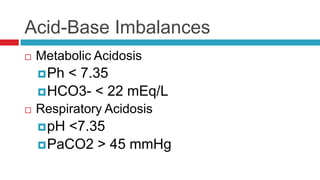
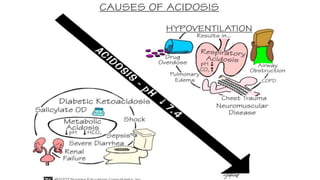
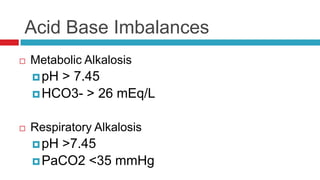
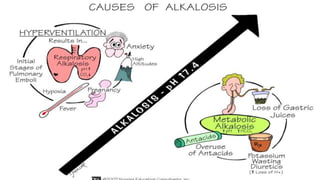
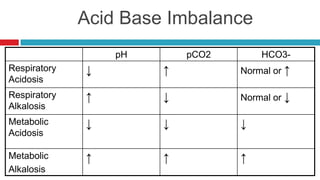
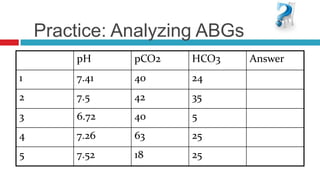
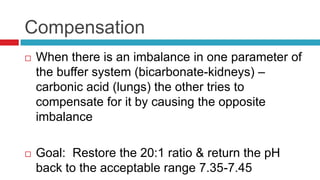
The document discusses acid-base balance and acid-base disorders. It describes three main systems that help maintain pH balance - buffers, the respiratory system, and the renal system. It explains how to interpret arterial blood gases by evaluating the pH, pCO2, HCO3, and other values to determine if a patient has respiratory or metabolic acidosis or alkalosis. Compensation by other systems is discussed when one system is imbalanced. Interpreting values and identifying primary vs compensated disorders is key to proper nursing care.