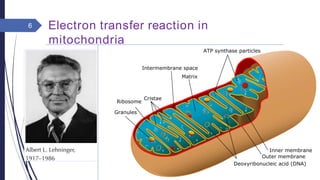
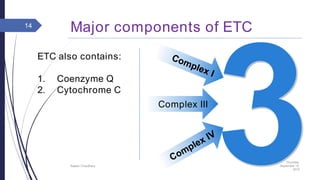
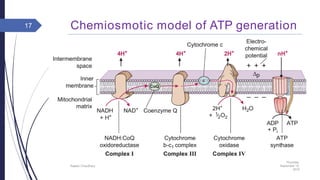
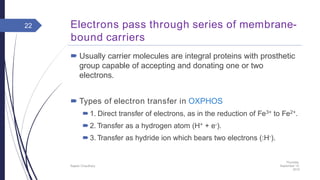
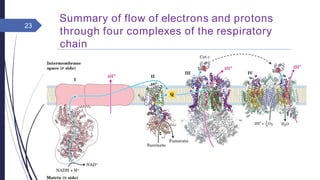
This document summarizes oxidative phosphorylation (OXPHOS) and the electron transport chain in mitochondria. It states that OXPHOS is essential for generating ATP through the transfer of electrons from donor molecules like NADH to oxygen. It occurs through five protein complexes embedded in the inner mitochondrial membrane: complex I-IV transfer electrons and pump protons out of the matrix, while complex V uses the proton gradient to drive ATP synthesis. The document provides an overview of each complex and how they facilitate electron transfer and proton pumping to create the electrochemical gradient used for ATP production.