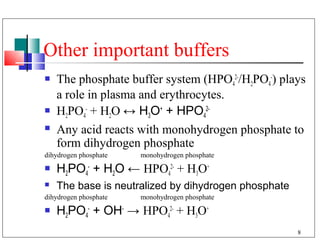
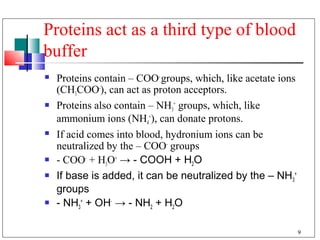
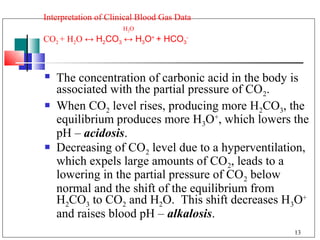
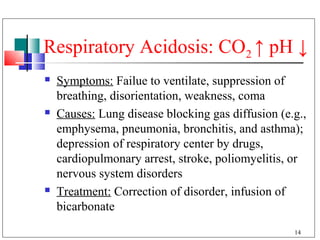
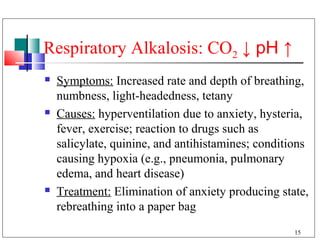
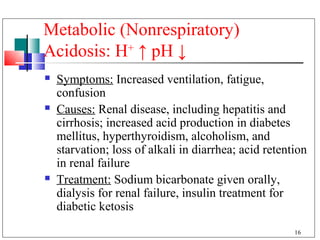
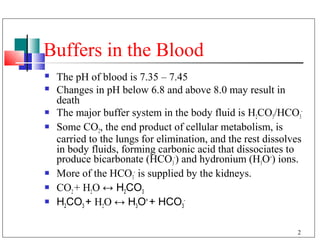
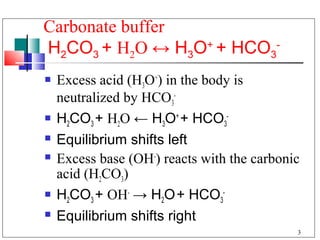
The major buffer system in the blood is the bicarbonate buffer system (H2CO3/HCO3-). Carbon dioxide produced from cellular metabolism dissolves in the blood to form carbonic acid, which dissociates into bicarbonate and hydronium ions. The lungs and kidneys help regulate blood pH by controlling the levels of carbon dioxide and bicarbonate. Disruptions to this buffer system can result in acidosis or alkalosis, which have various causes and symptoms that require different treatments.
![pH of the blood buffer
The concentrations in the blood of H2CO3 and
HCO3- are 0.0024M and 0.024 respectively
H2CO3/ HCO3- = 1/10 is needed to maintain the
normal blood pH (7.35 – 7.45) −
[ H 3O + ][ HCO3 ]
Ka =
[ H 2CO3 ]
[ H 2CO3 ]
[ H 3O + ] = K a −
=
[ HCO3 ]
0.0024
= 4.3 x10 −7 x = 4.3 x10 −7 x0.10 = 4.3 x10 −8
0.024
pH = − log(4.3 x10 −8 ) = 7.37
4](https://image.slidesharecdn.com/bufferintheblood-130115121113-phpapp02/85/Buffer-in-the-blood-4-320.jpg)