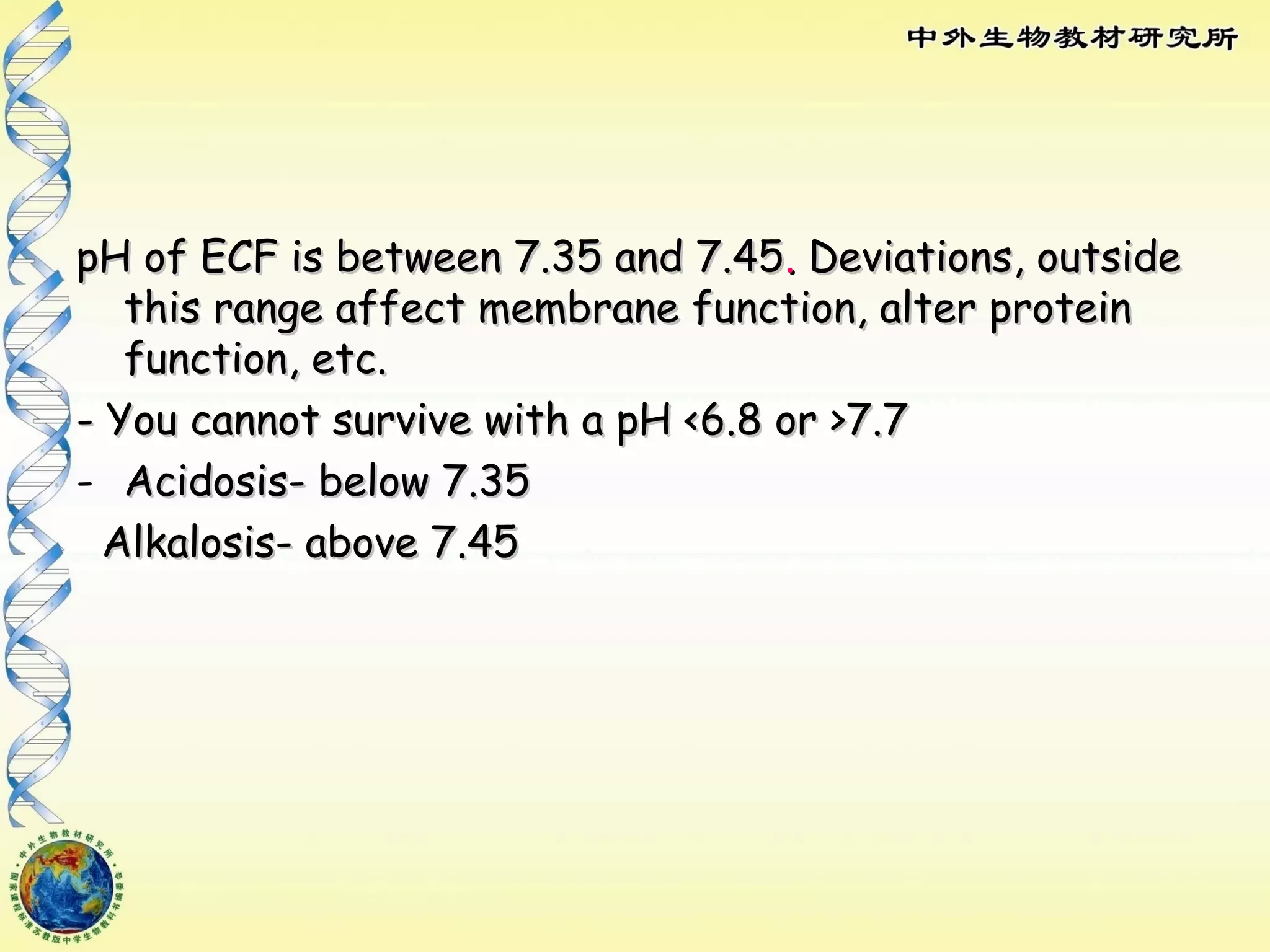
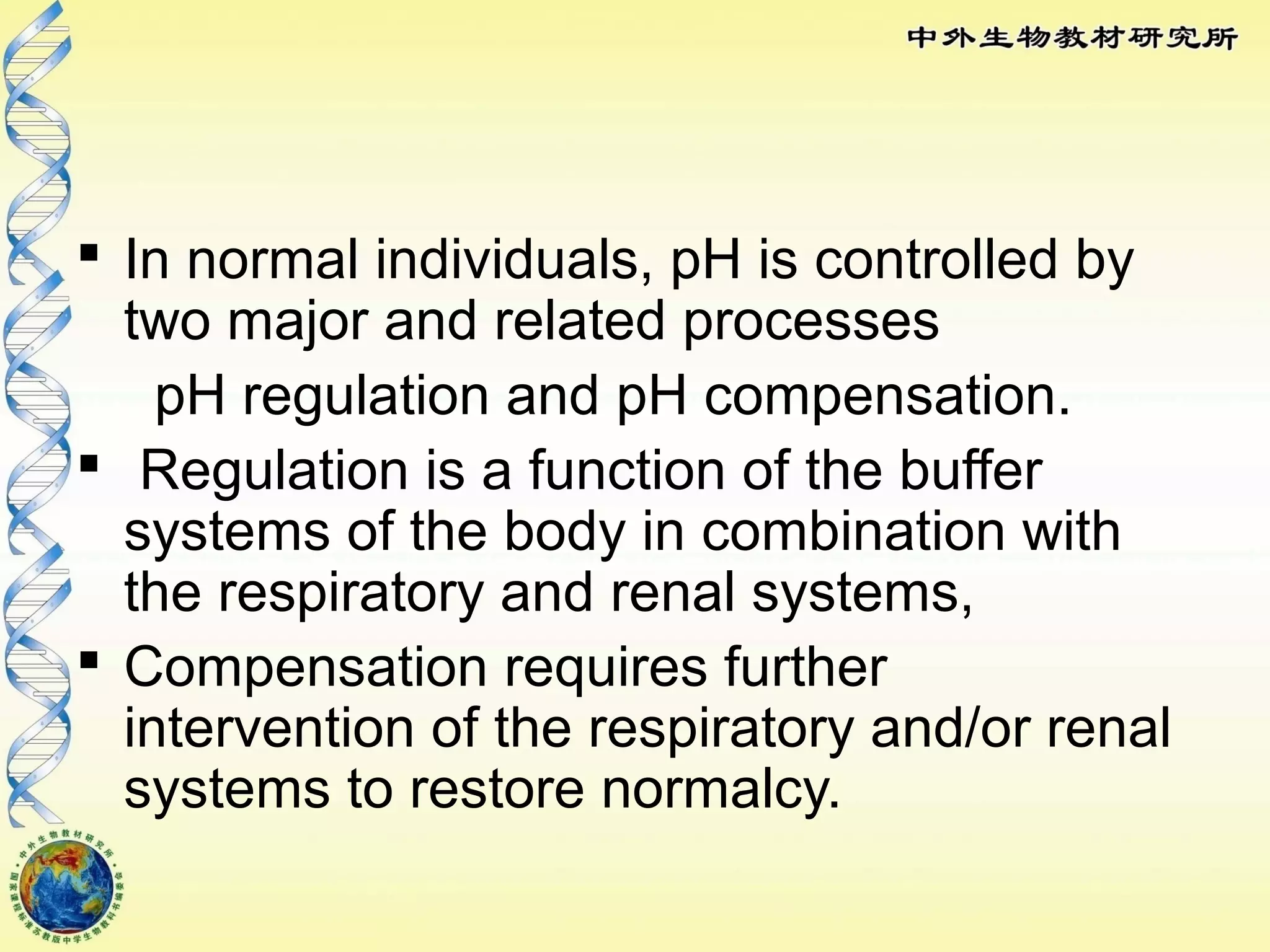
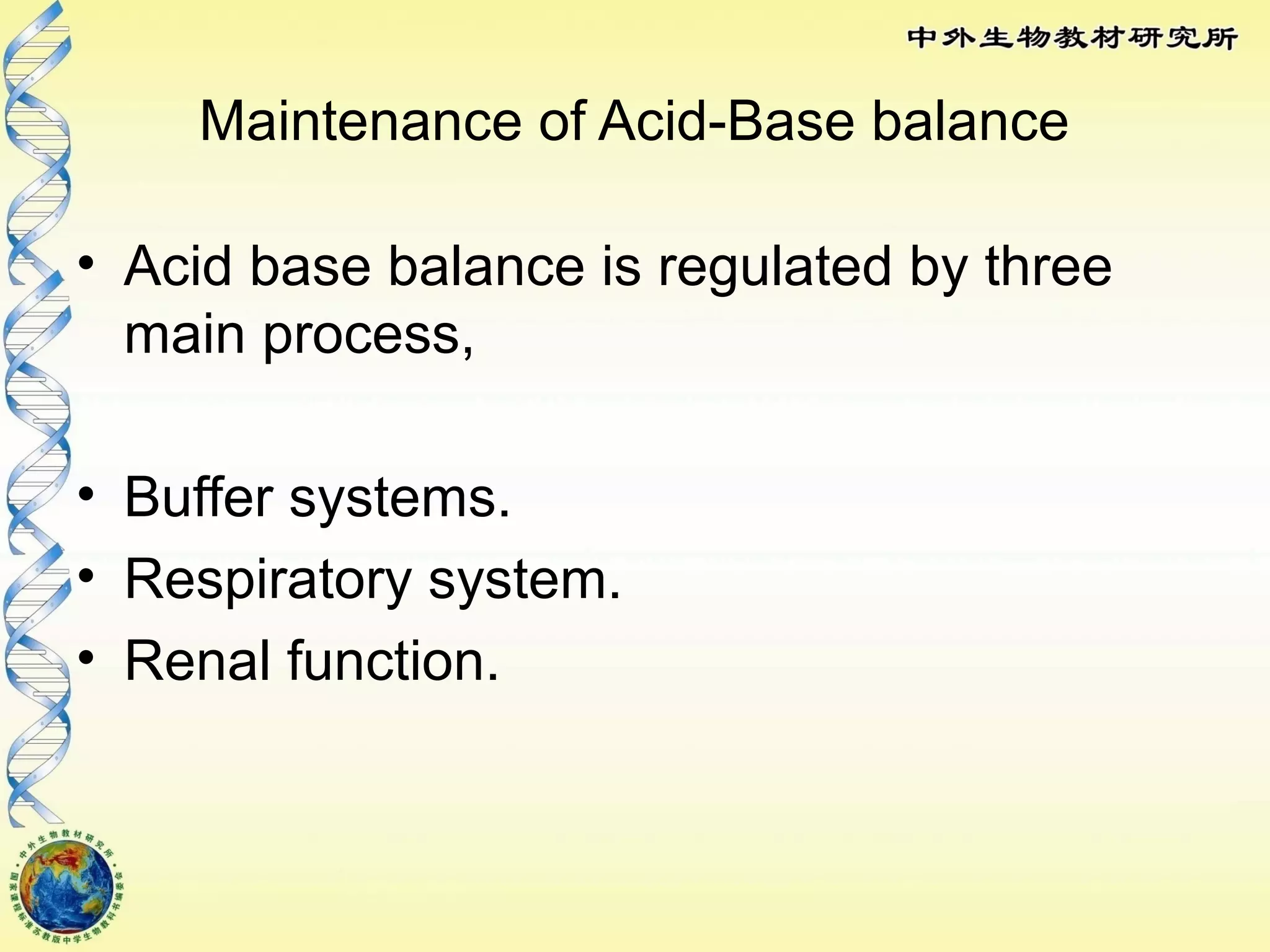
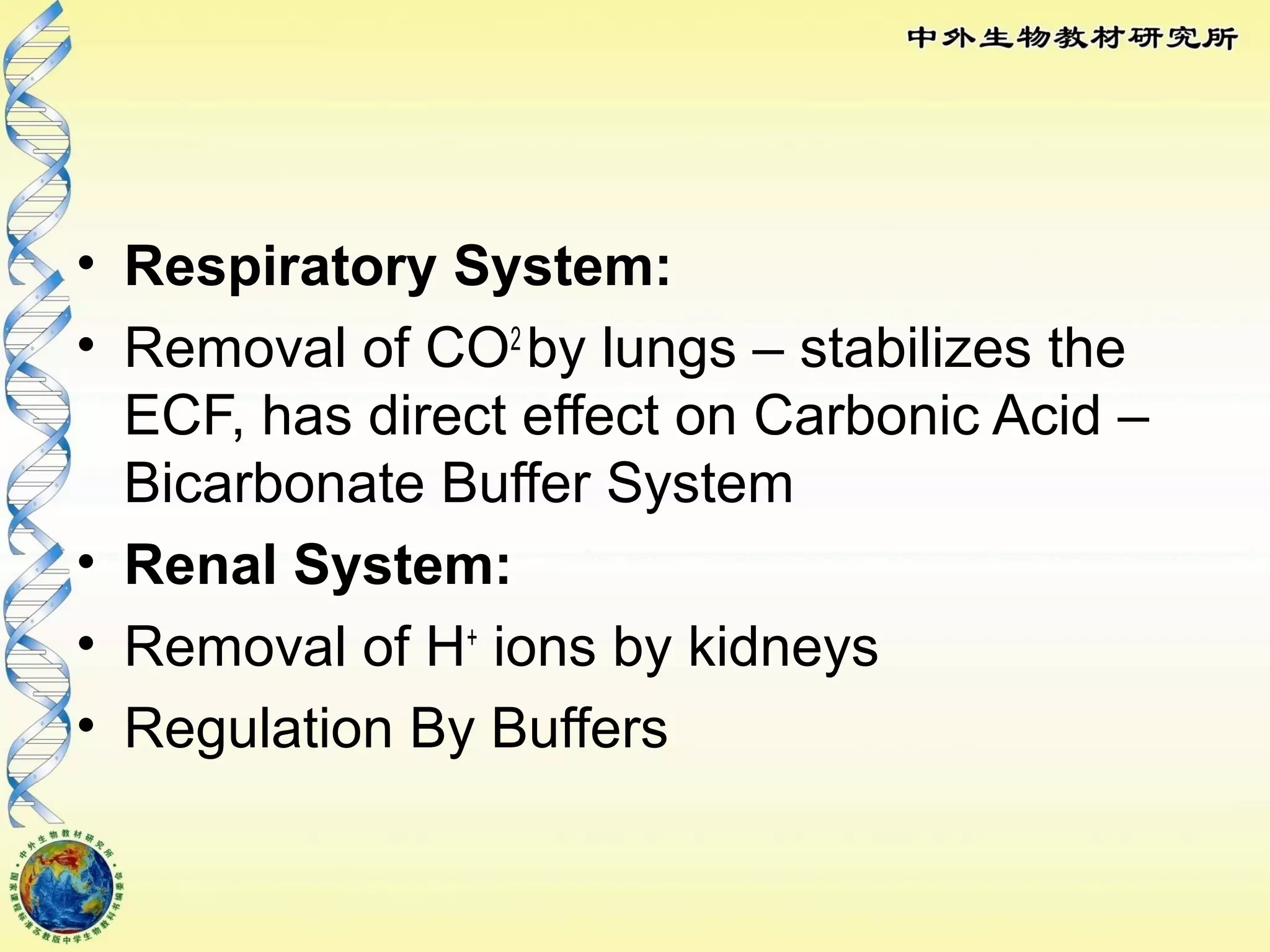
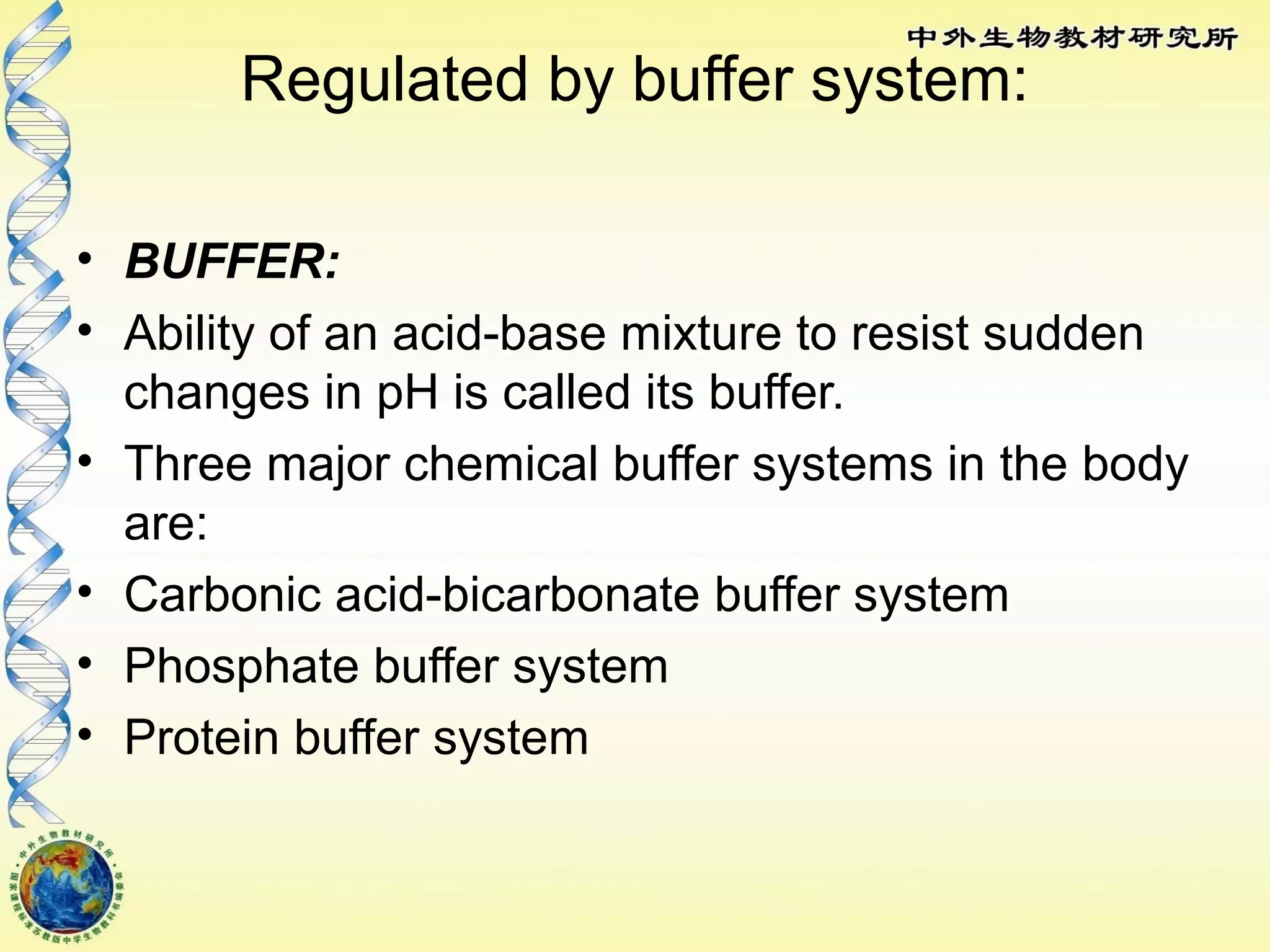
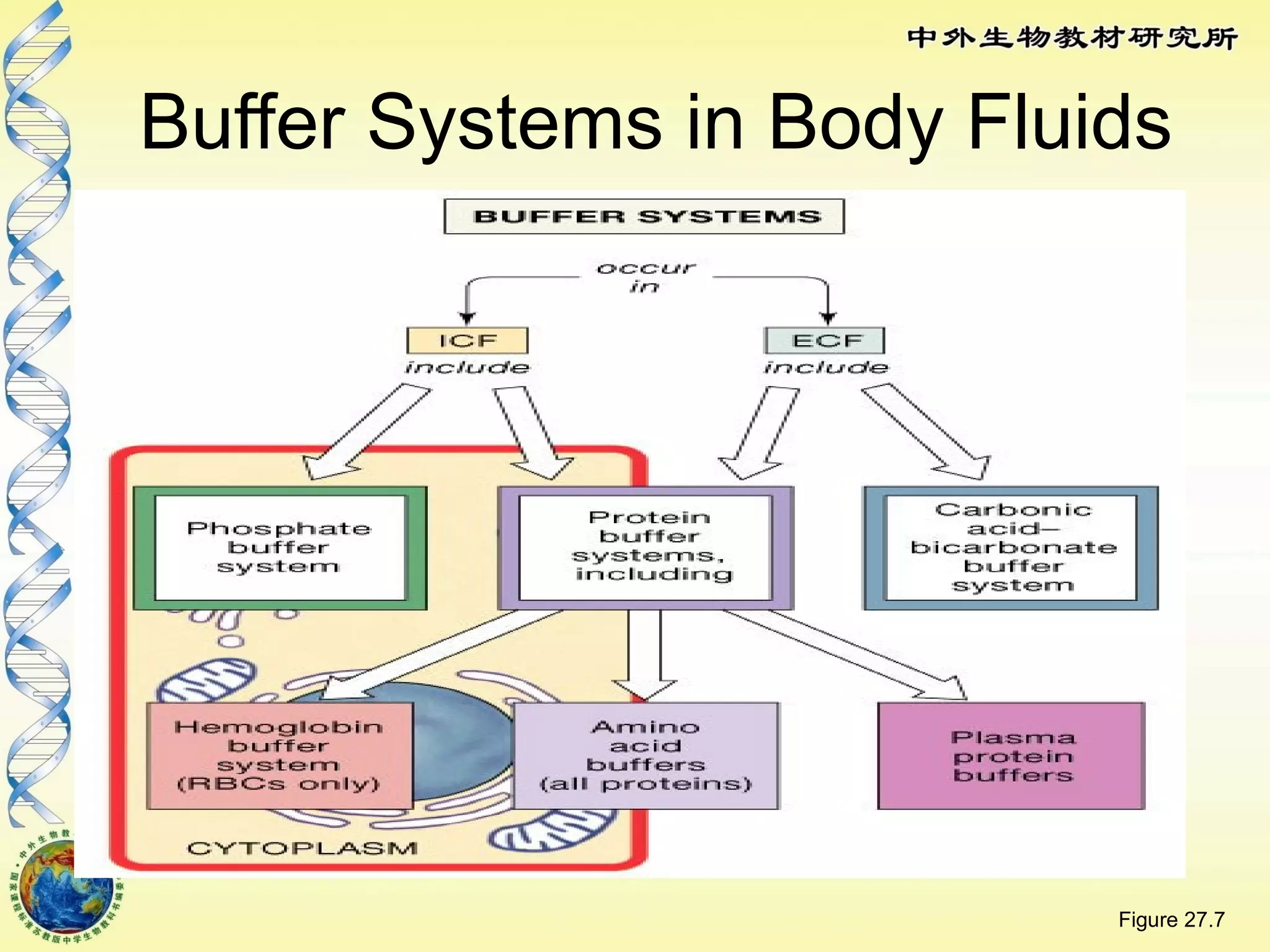
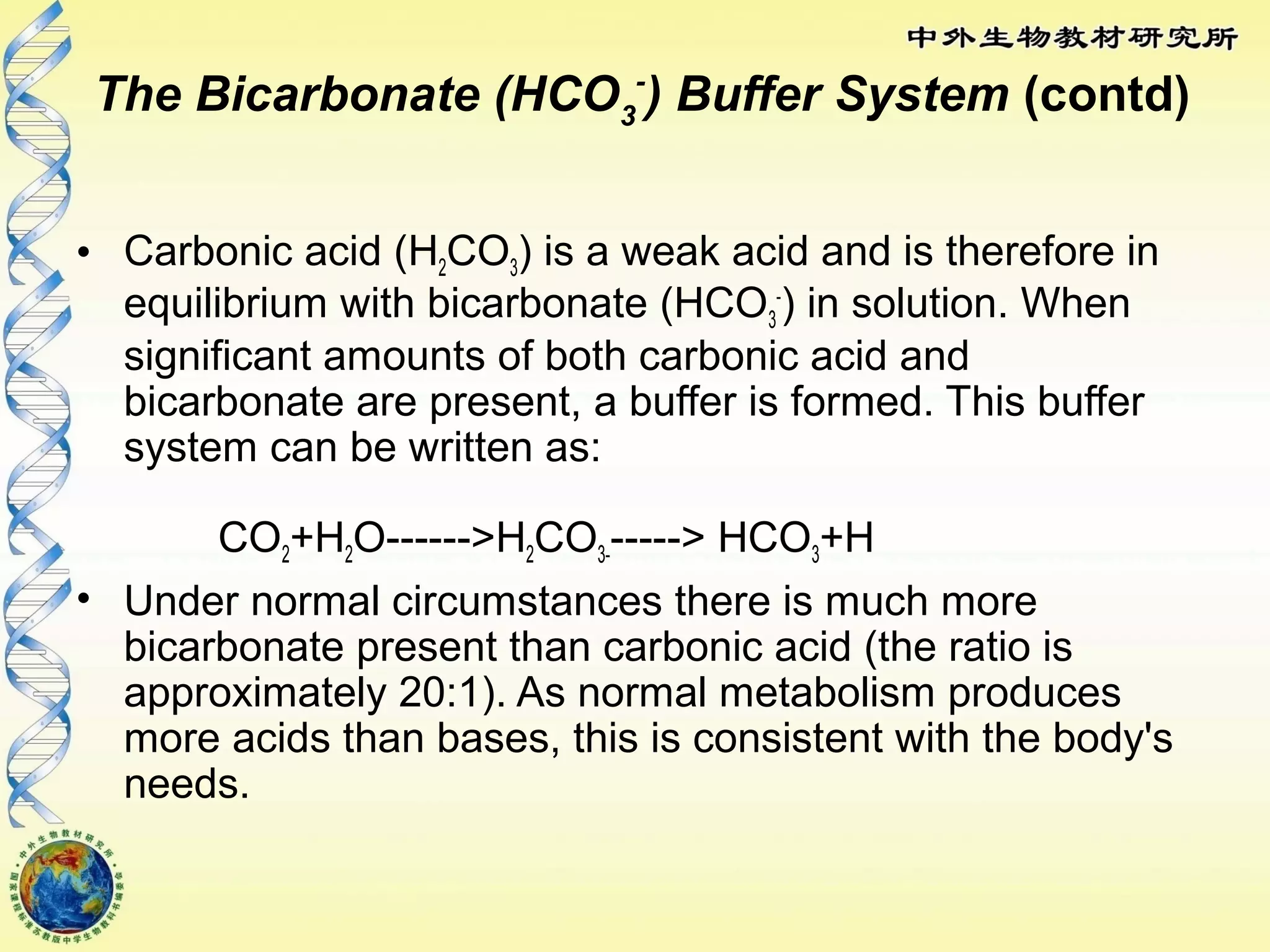
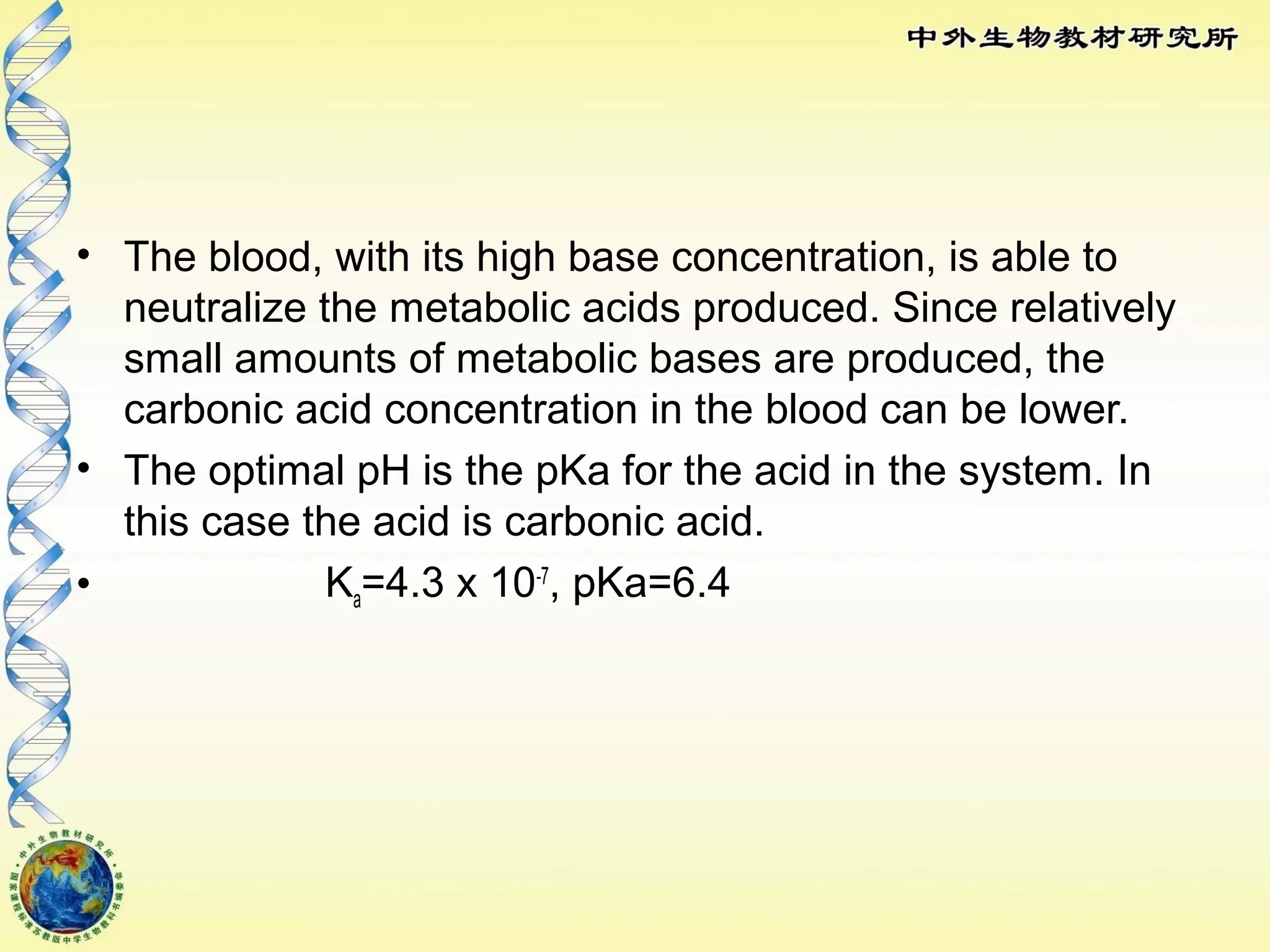
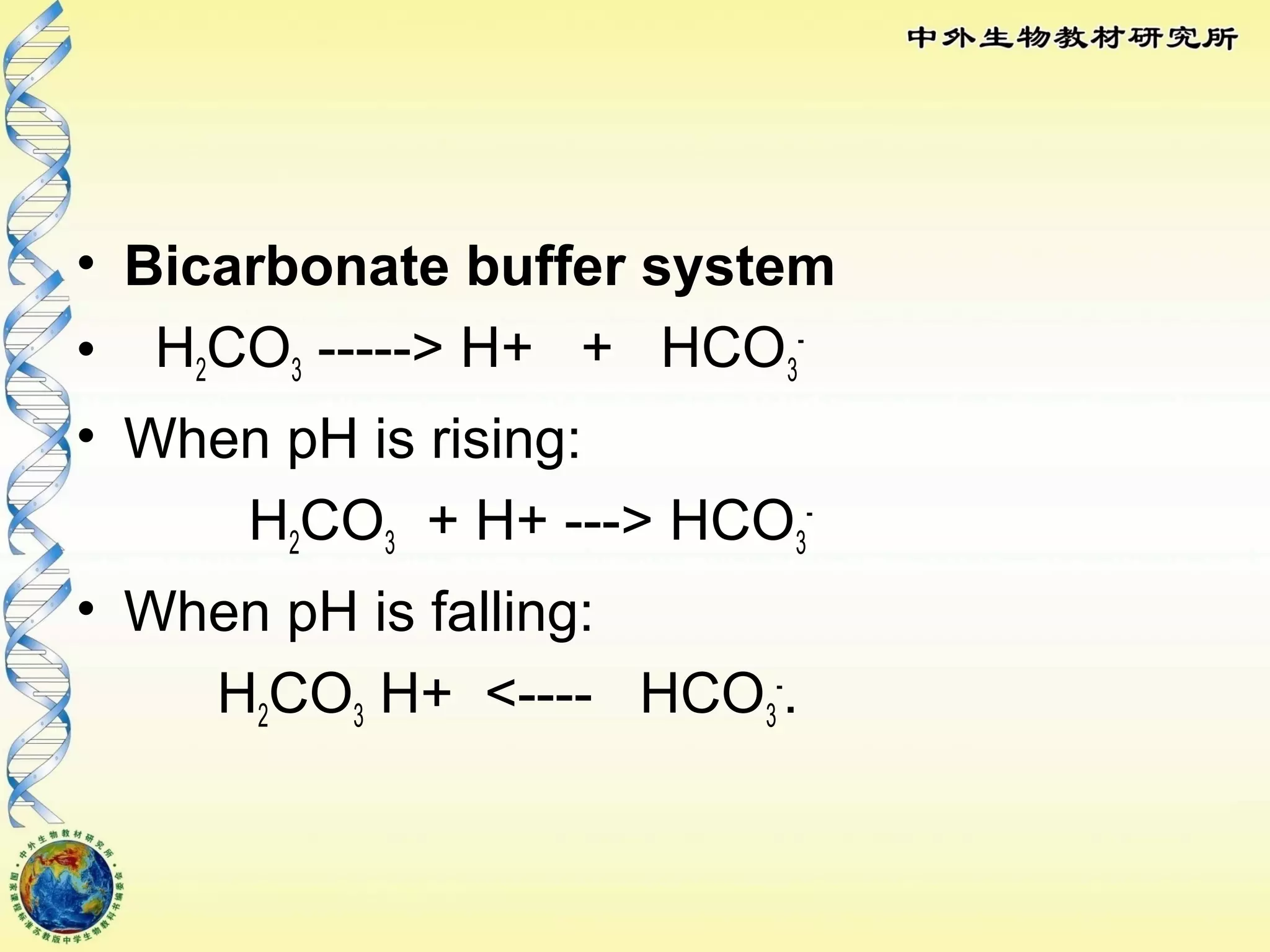
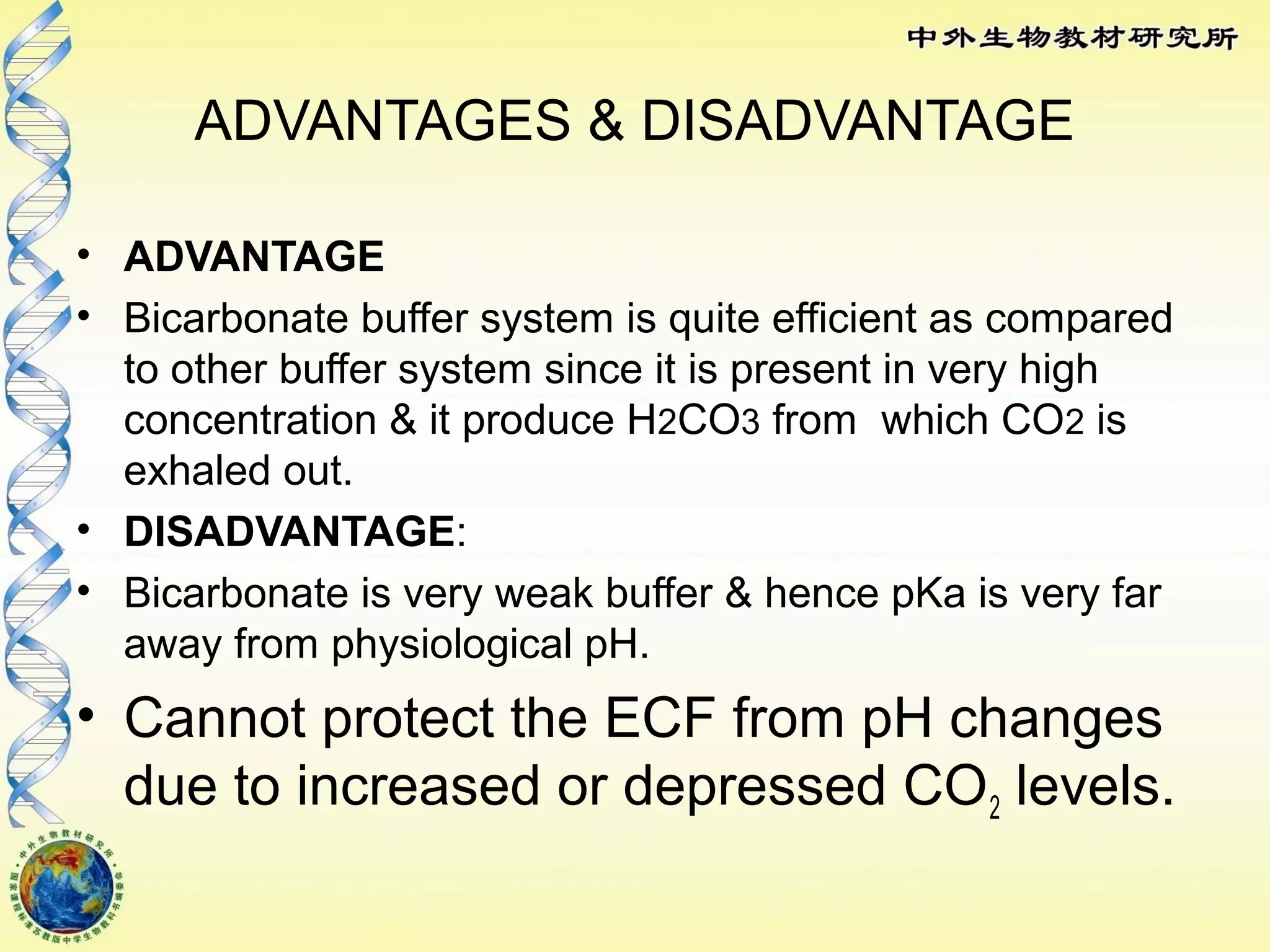
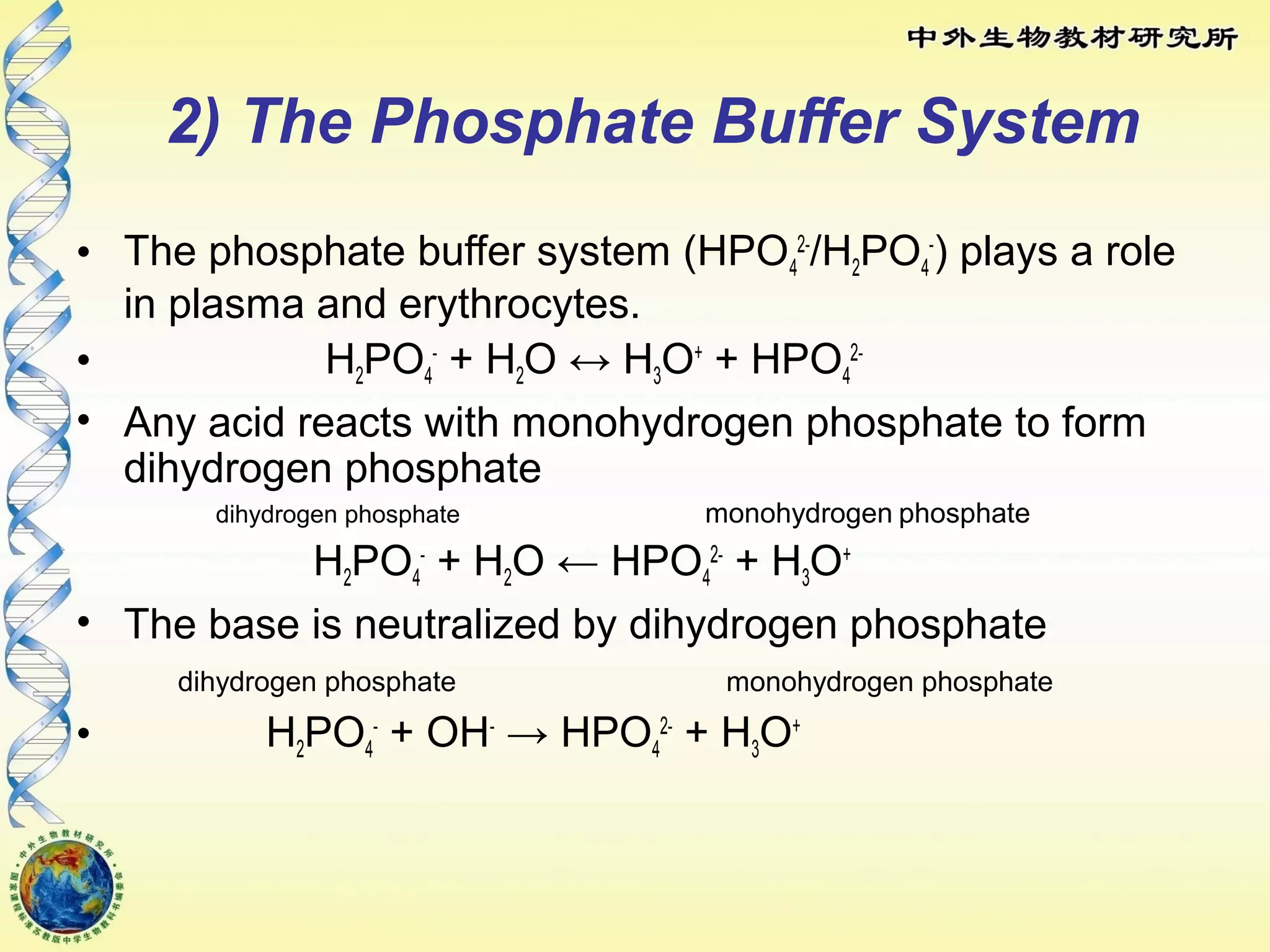
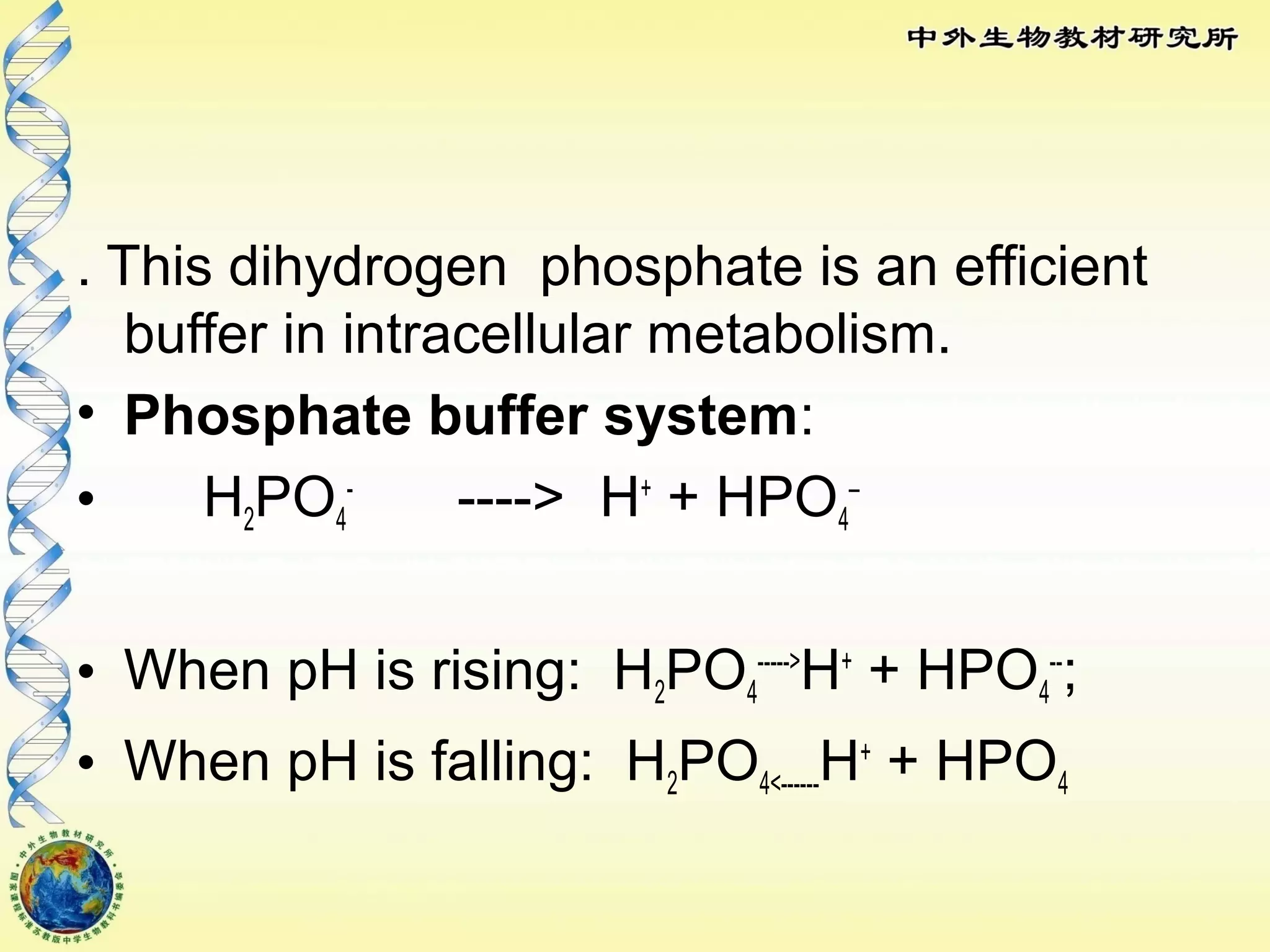
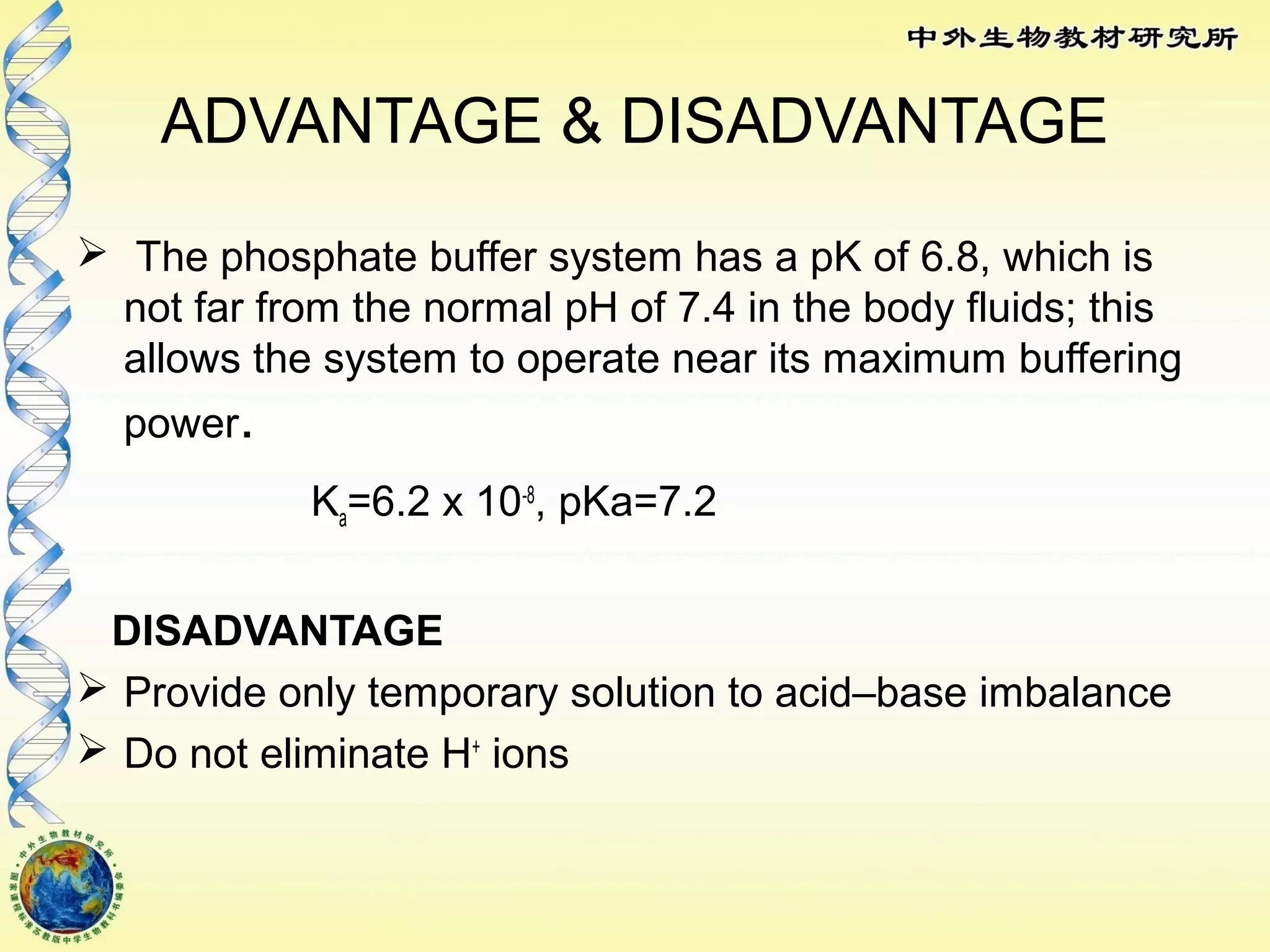
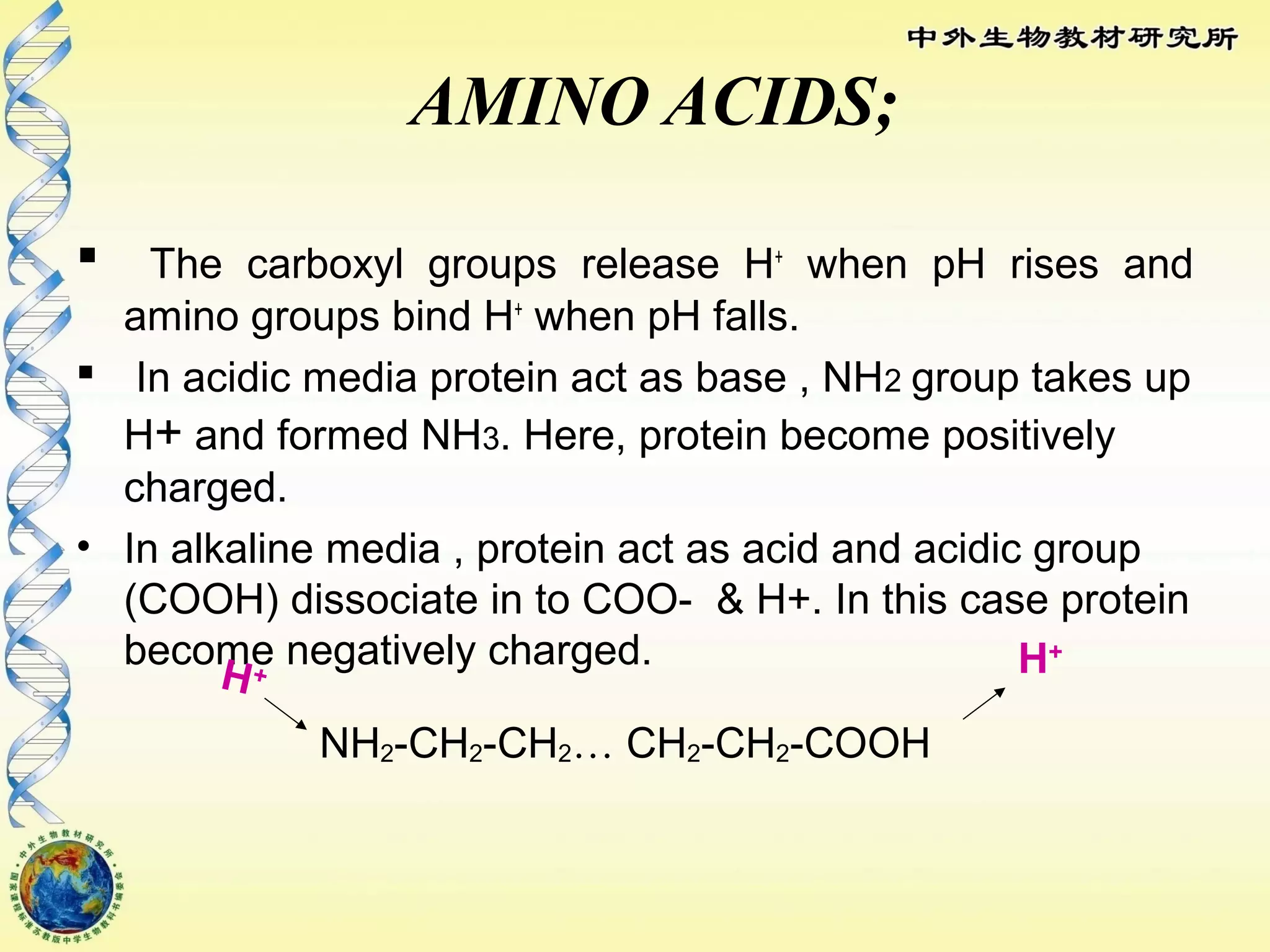
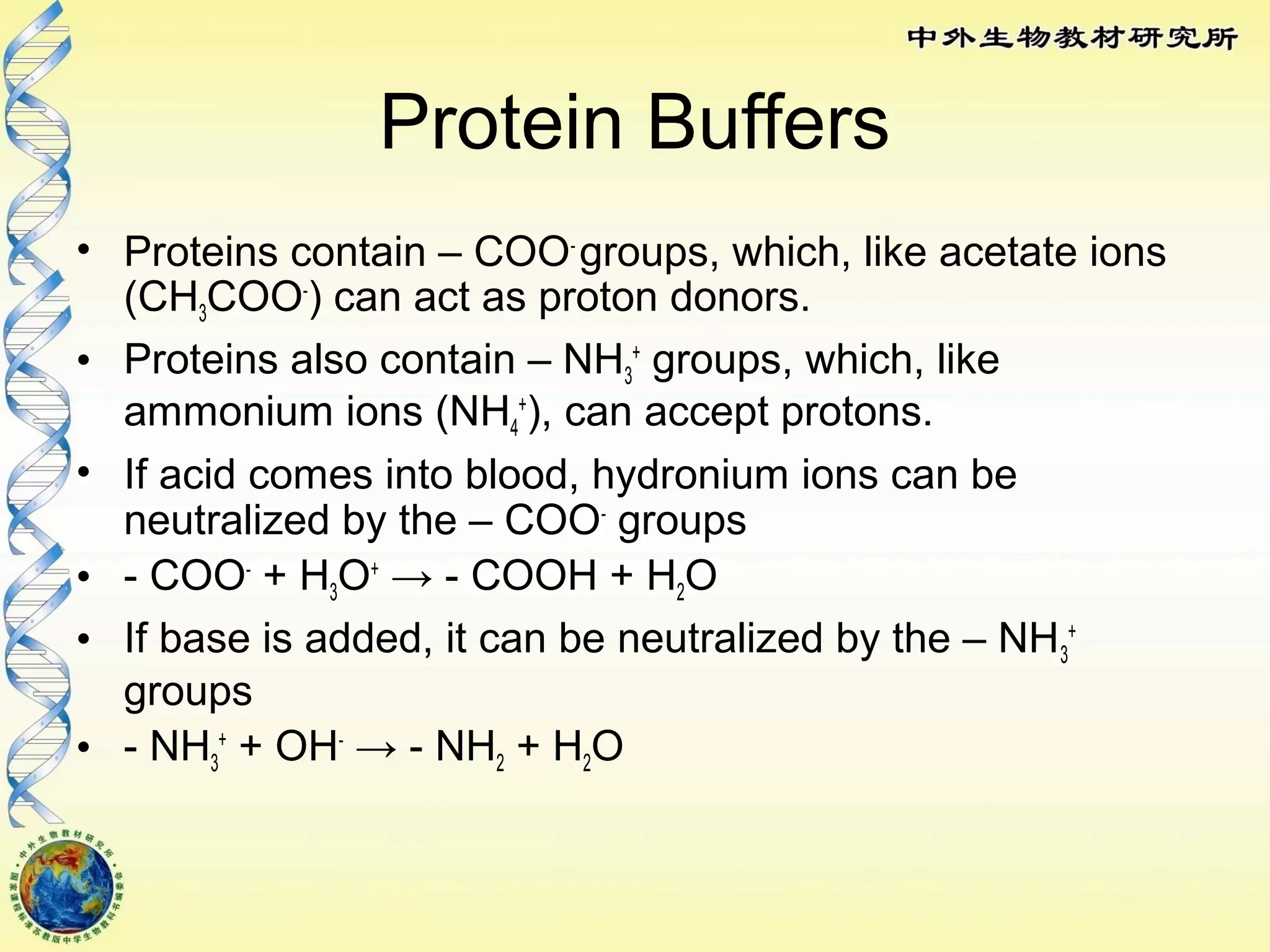
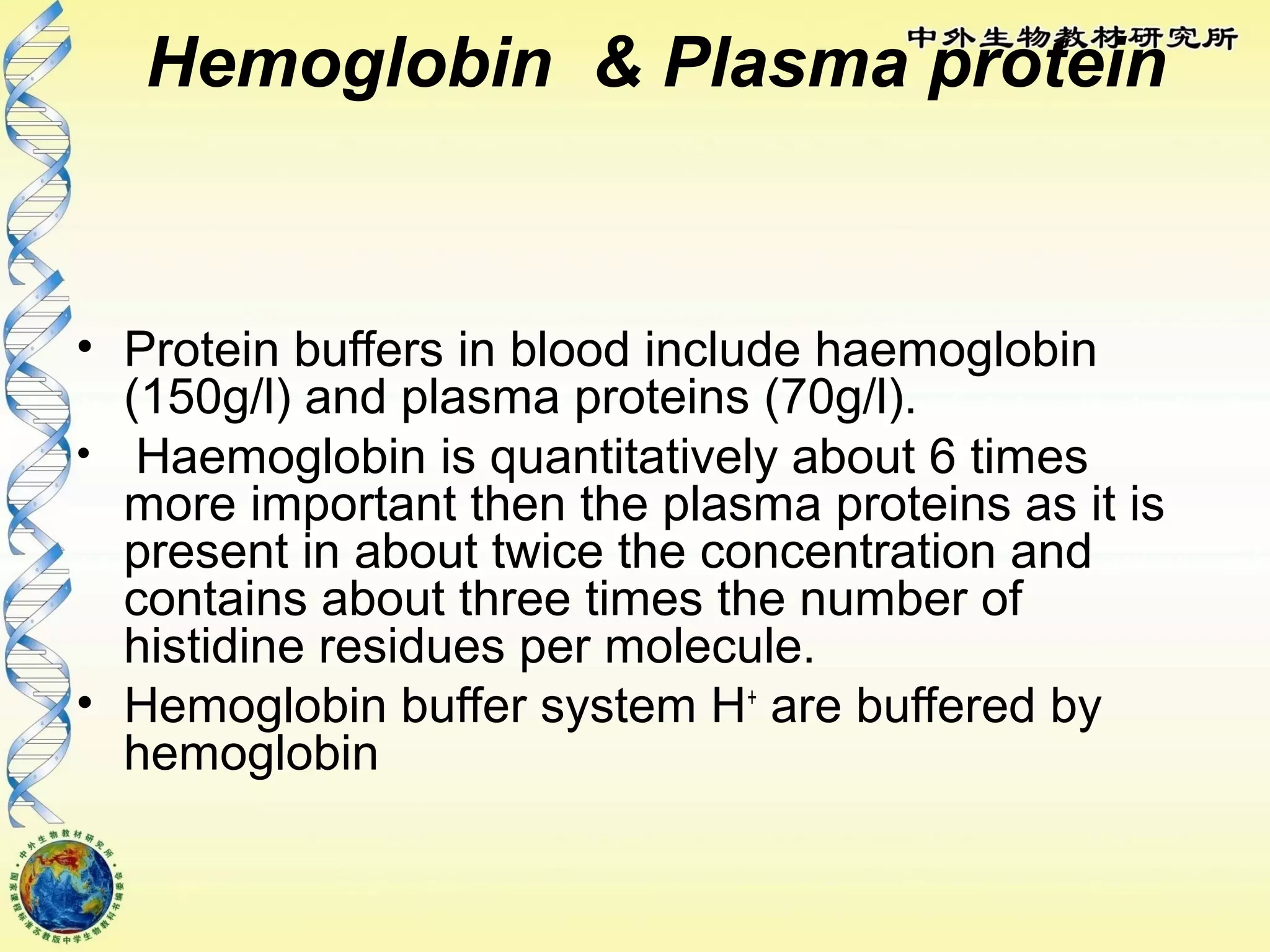
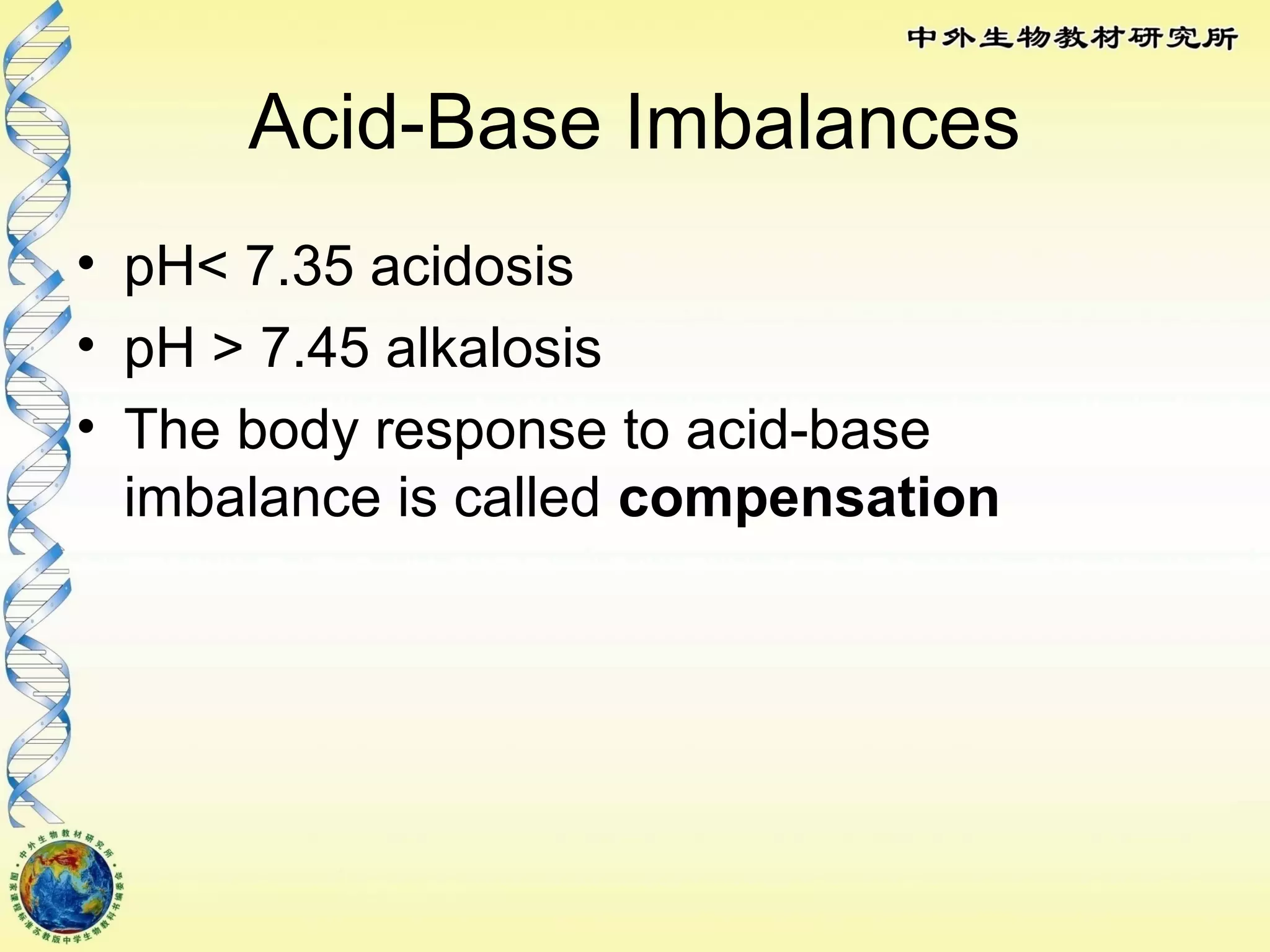
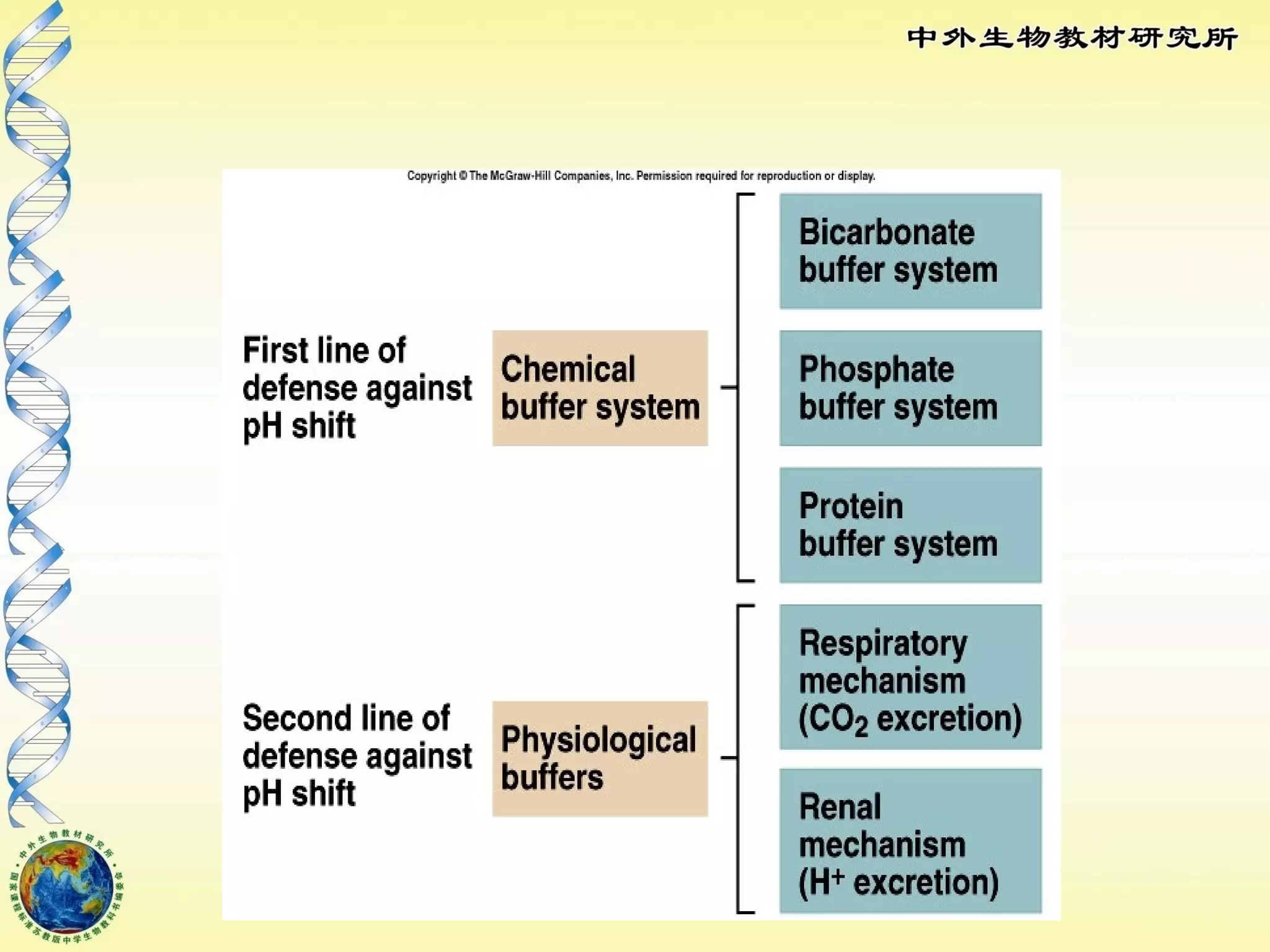
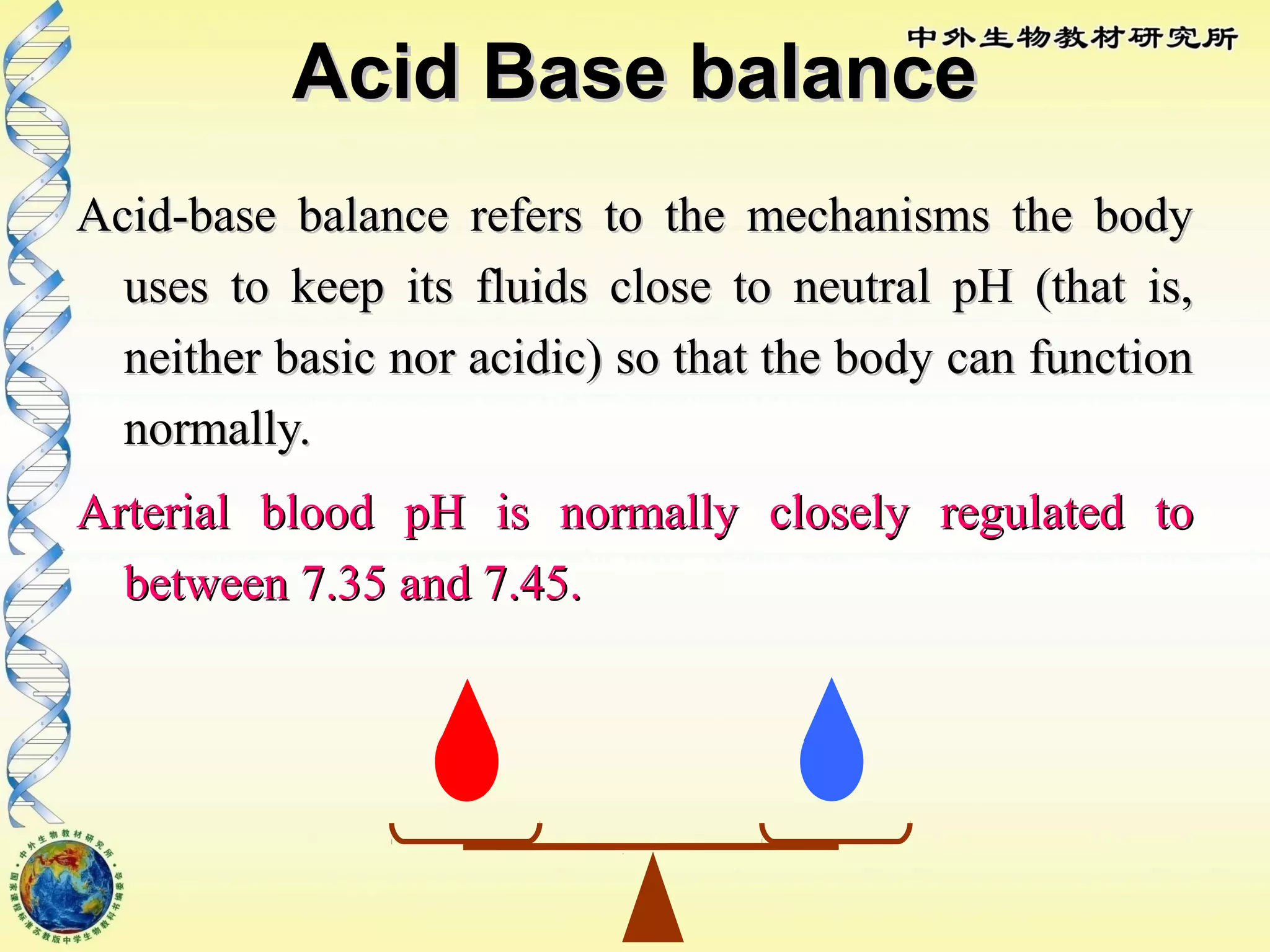
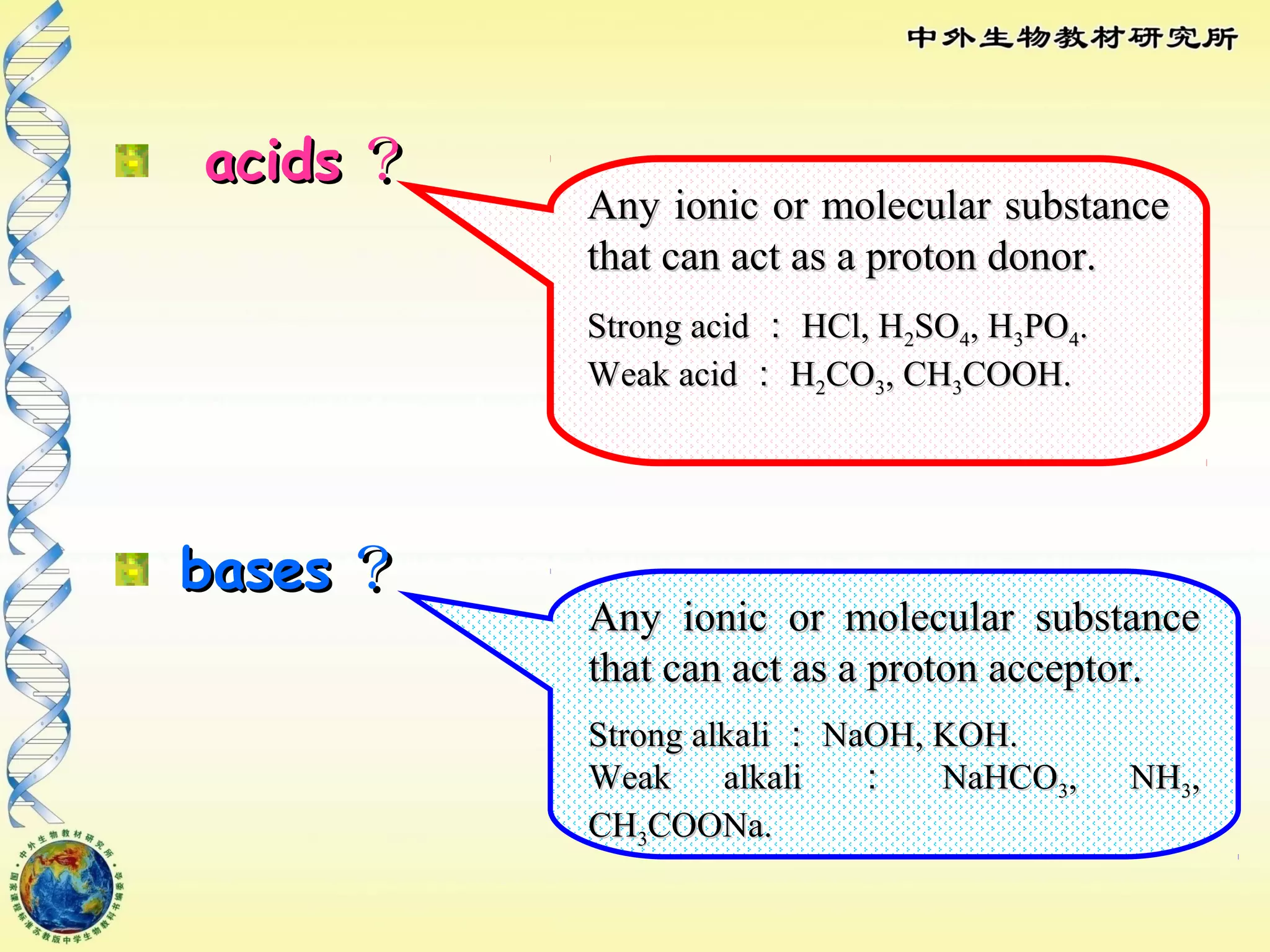
The body maintains tight regulation of arterial blood pH between 7.35-7.45 through acid-base balance mechanisms. It uses buffer systems, and respiratory and renal systems to neutralize acids and bases. The major buffer systems are bicarbonate, phosphate, and proteins, which maintain pH by donating or accepting hydrogen ions. Deviations outside the normal pH range can impair membrane and protein function and are not survivable. The lungs and kidneys work to restore pH through removing carbon dioxide and hydrogen ions respectively.
![pH
• pH is the negative log of hydrogen ion
concentration.
pH= -log[H+]
If [H+
] is high, the solution
is acidic; pH < 7
If [H+
] is low, the solution
is basic or alkaline ; pH > 7](https://image.slidesharecdn.com/acidbasebalance-140621084212-phpapp01/75/Acid-base-balance-summary-3-2048.jpg)