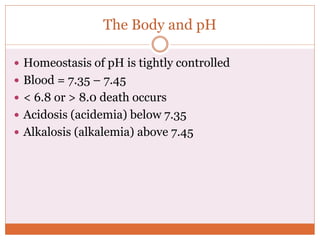
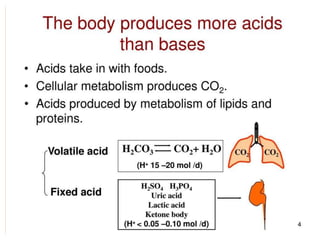
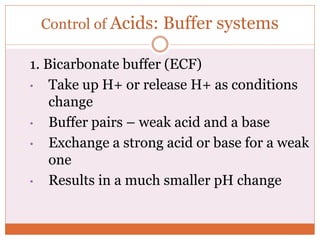
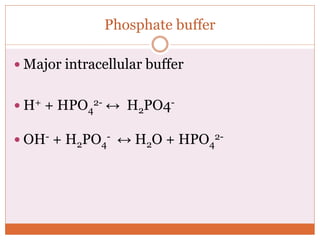
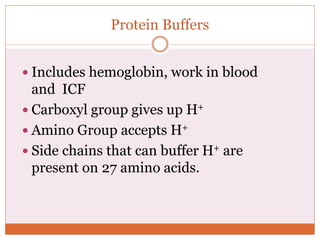
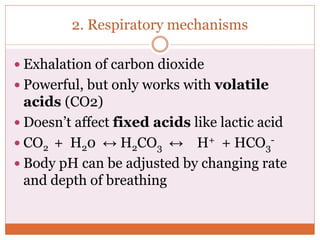
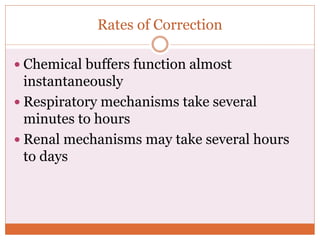
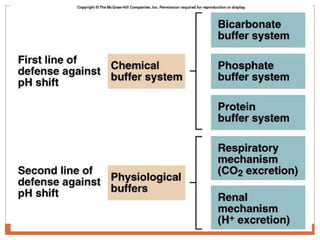
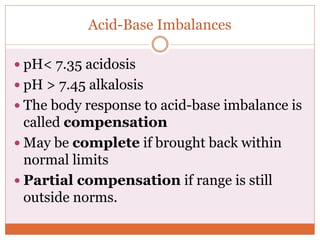
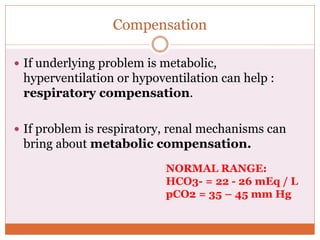
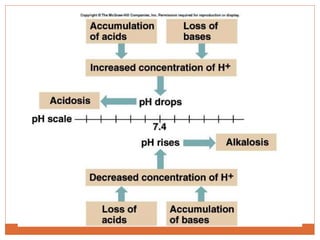
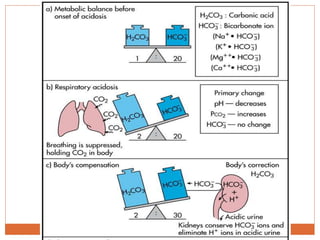
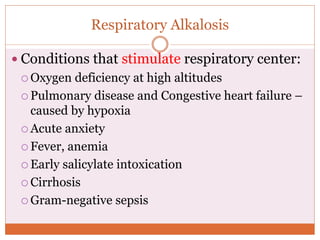
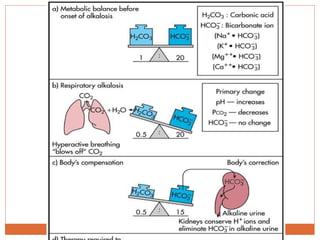
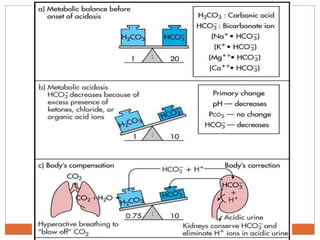
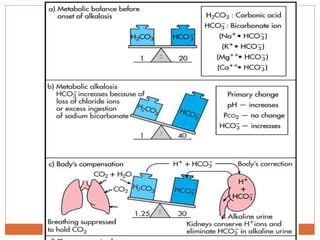
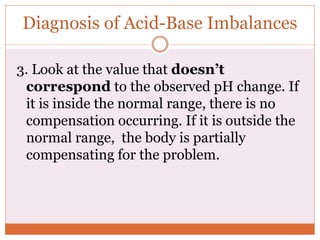
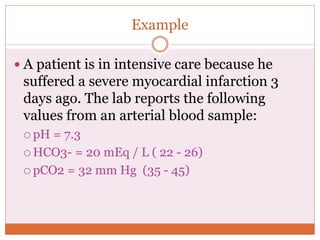
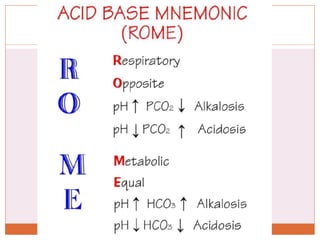
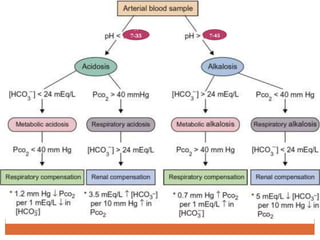
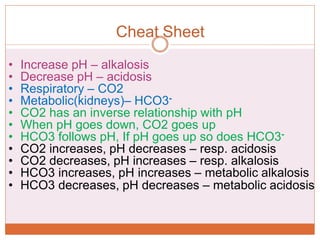
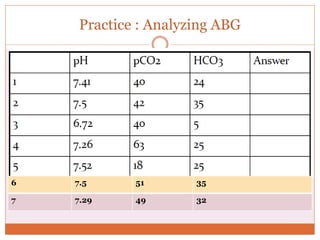
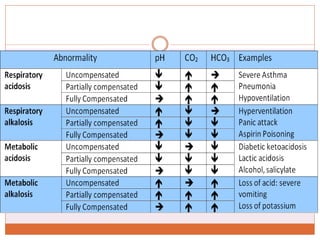
The document discusses acid-base balance and imbalances in the human body. It explains that the body tightly regulates blood pH between 7.35-7.45 through buffer systems, respiration, and kidney function. When pH falls below or rises above this range, acidosis or alkalosis occurs respectively. Various conditions can cause respiratory or metabolic acidosis/alkalosis by affecting carbon dioxide or bicarbonate levels. The body attempts to compensate for imbalances through opposing respiratory or renal responses depending on the underlying cause. Diagnosis involves determining if the pH, carbon dioxide, or bicarbonate levels are abnormal and whether compensation is partial or complete.