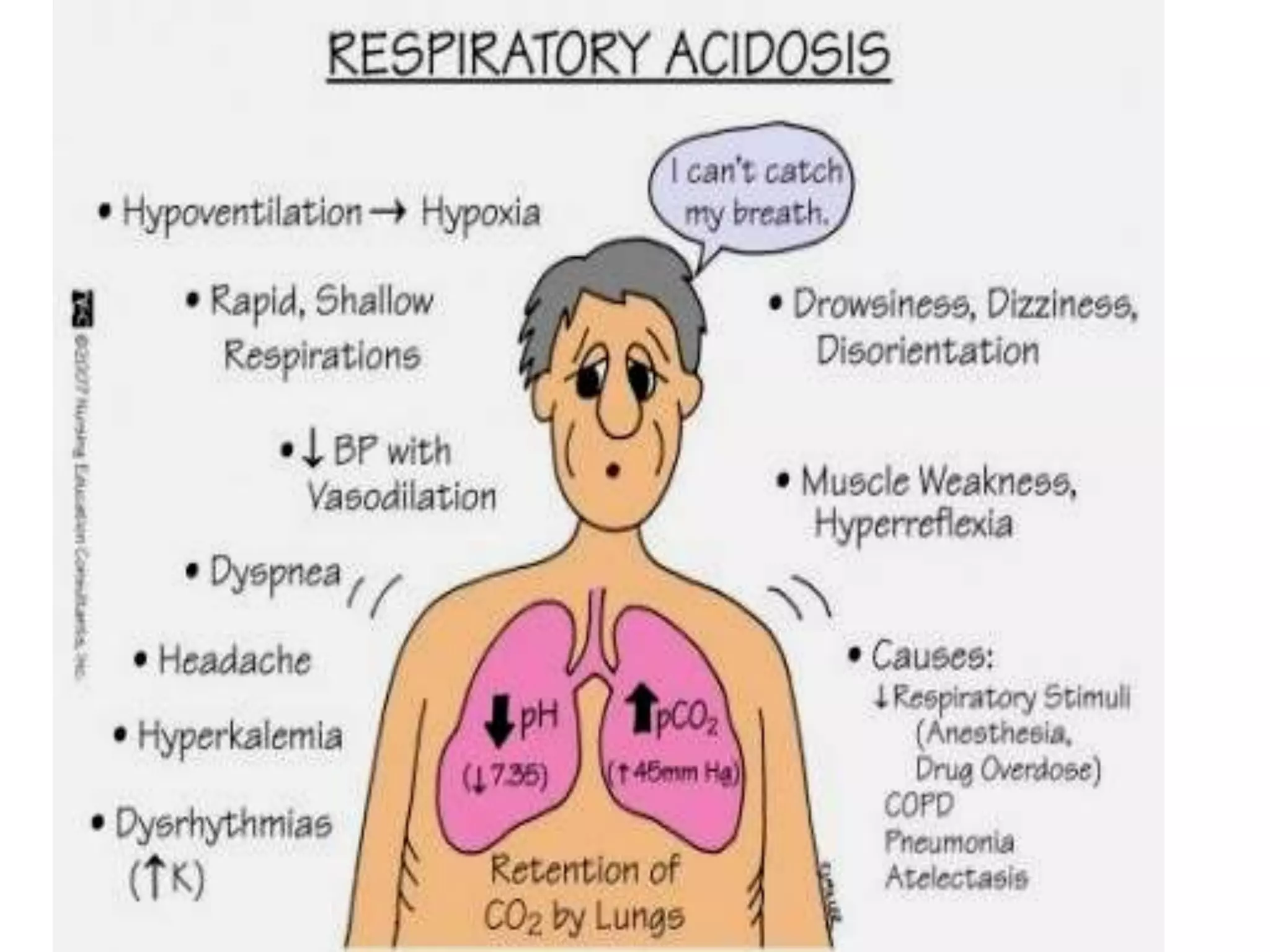
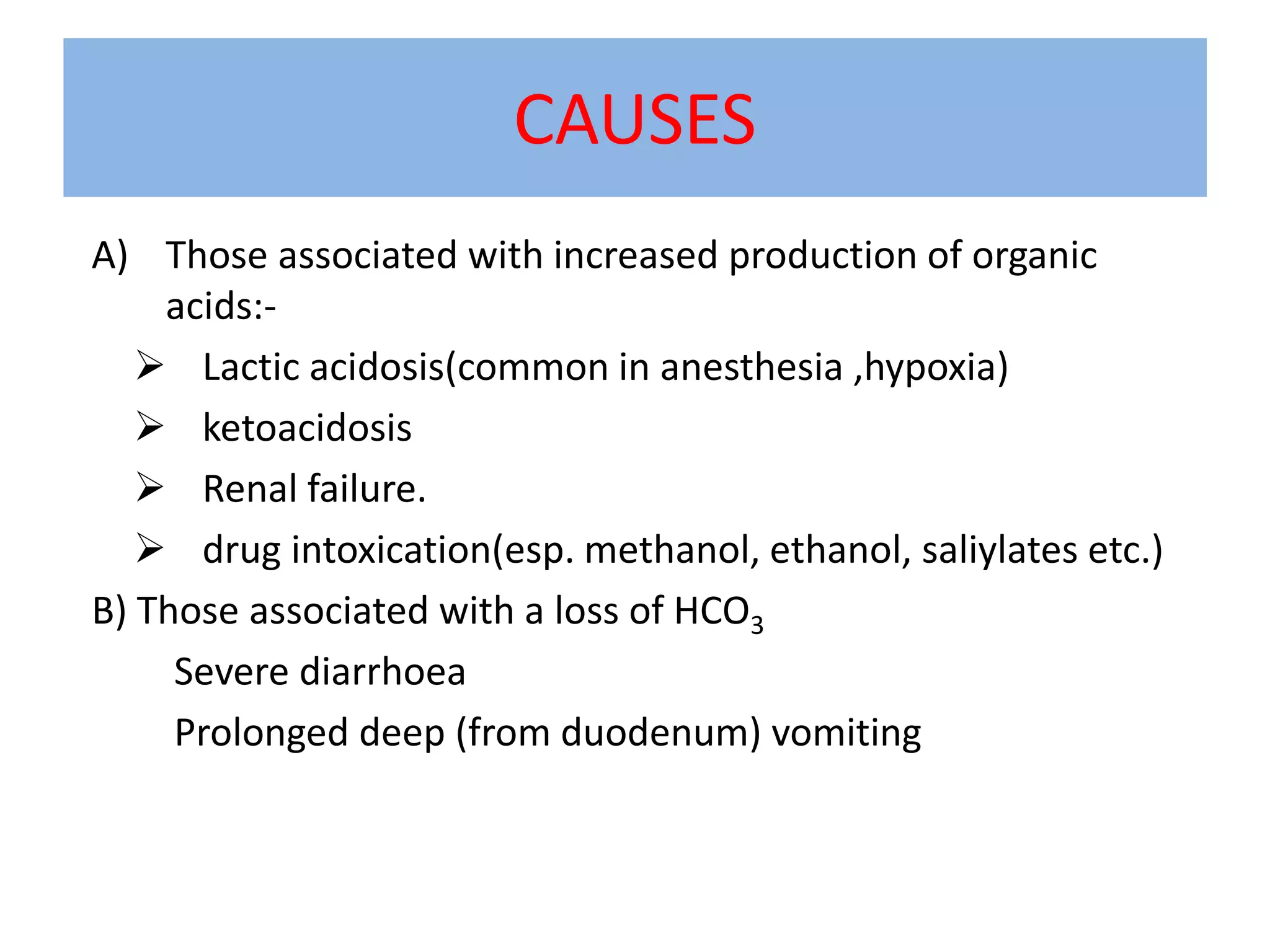
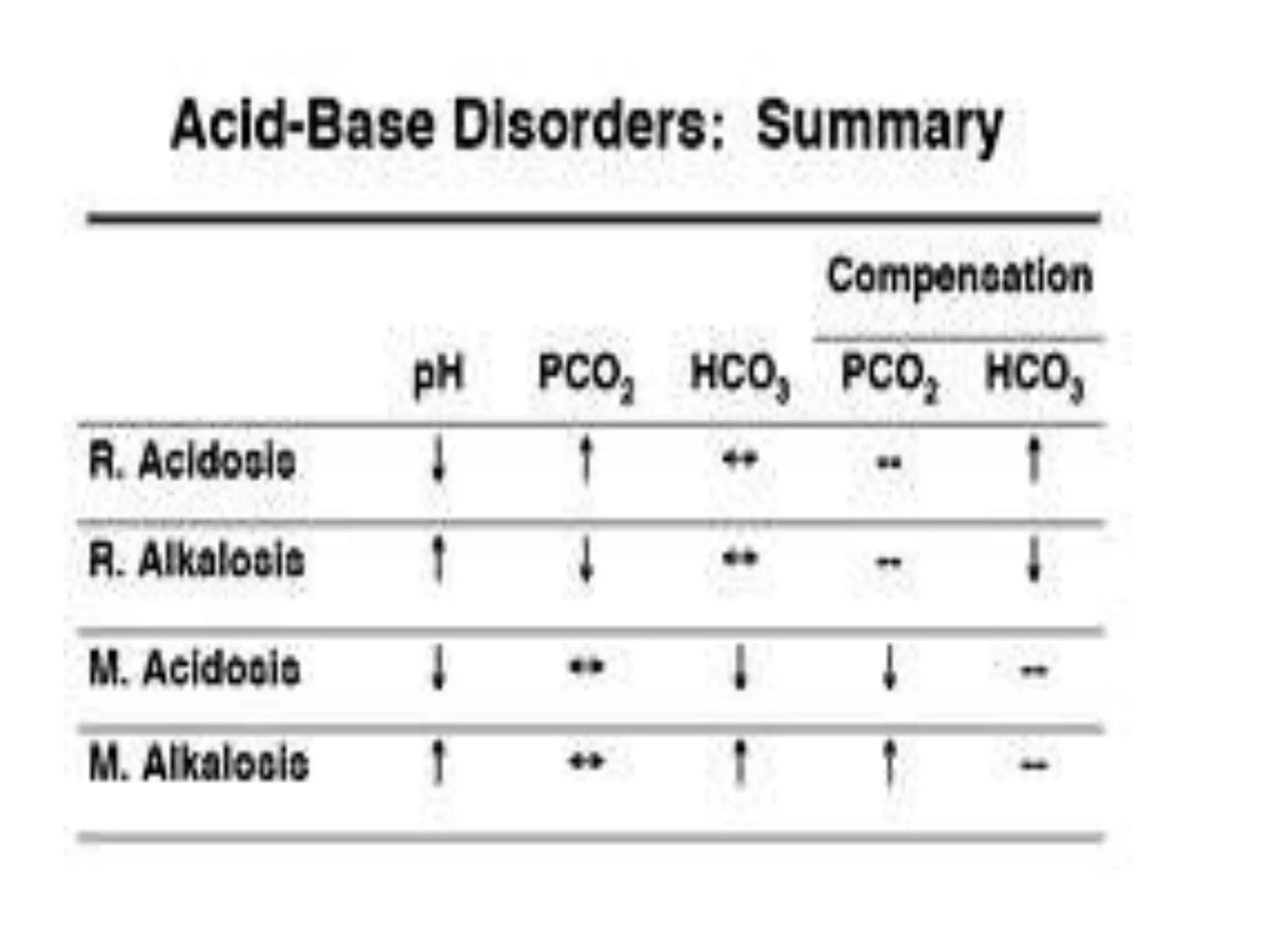
This document discusses acid-base disorders and ABG analysis. It defines acids and bases, describes the mechanisms that regulate acid-base balance, and classifies different types of acid-base imbalances including respiratory acidosis, respiratory alkalosis, metabolic acidosis, and metabolic alkalosis. Causes, compensatory mechanisms, and treatments are provided for each type of imbalance. The document also covers ABG analysis, normal values, interpretation of results, and provides two sample problems to identify acid-base disturbances.





![• pH of a buffer system may be determined from
the Henderson- Hasselbach equation :
pH=pK+log10([HCO3
-]/[H2CO3])
7](https://image.slidesharecdn.com/acidbase-170306102247/75/acid-base-disorder-and-ABG-analysis-7-2048.jpg)